Reactions of Alcohols and Ethers: Overview and Mastering Key Topics
In this lecture, students delve into reactions of alcohols and ethers, including oxidation, dehydration, esterification, and alkaline hydrolysis. Master skills like identifying alcohols and understanding key tests. Explore oxidation-reduction reactions, review functional groups, and learn essential mechanisms. Dive into examples, practice questions, and understand the reactions in-depth.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
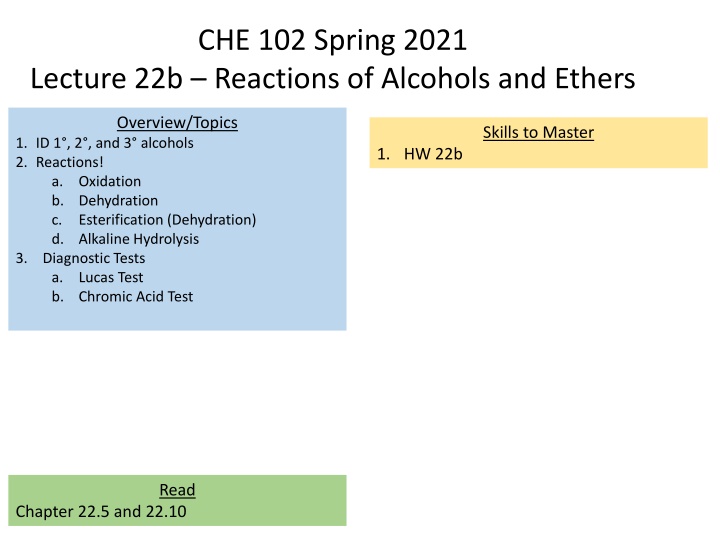
CHE 102 Spring 2021 Lecture 22b Reactions of Alcohols and Ethers Overview/Topics Skills to Master 1. ID 1 , 2 , and 3 alcohols 2. Reactions! a. Oxidation b. Dehydration c. Esterification (Dehydration) d. Alkaline Hydrolysis 3. Diagnostic Tests a. Lucas Test b. Chromic Acid Test 1. HW 22b Read Chapter 22.5 and 22.10
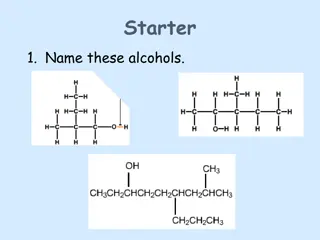
Review Topics 1. Primary/Secondary/Tertiary Alcohols 2. Saytzeff s Rule 3. Substitution Reactions 4. Elimination Reactions 5. Functional Groups (next slide) 6. Oxidation/Reduction (next slide)
Review Functional Groups Aldehyde Ketone Carboxylic Acid (CA) Ester
Oxidation-Reduction Reactions Redox [O] Oxidation Reduction 1. Lose Electrons 2. Lose bonds to H 3. Gain bonds to O 4. Molecule loses energy 1. Gain Electrons 2. Gain bonds to H 3. Lose bonds to O 4. Molecule gains energy Future: Ch. 33-35
Oxidation Reactions [O] [O] 1 Alcohol Aldehyde + H2O Carboxylic Acid (CA) [O] [O] 2 Alcohol Ketone + H2O No Reaction (NR) [O] 3 Alcohol NR
Examples: Oxidation
Dehydration Reactions [-H2O] 1 Alcohol + 1 Alcohol Ether + H2O (Multiple Products Possible) [-H2O] 2 or 3 Alcohol Alkene + H2O ( Elimination Saytzeff s Rule) [H+] 1 Alcohol + CA Ester + H2O ( Esterfication ) [-H2O] 1 Alcohol + CA Ester + H2O
Examples: Dehydration 3 Types
Examples: Dehydration 3 types
Alkaline Hydrolysis Alkyl Halide + Strong Base Alcohol + Salt (Substitution or Na loves Cl ) Examples: You Try It:
Review Diagnostic Tests o Differentiate between Functional Groups o Normal Reactions associated with a visible change o Know what it tests for o Know what a + test looks like o Know what a test looks like o Write an example reaction
K2Cr2O7/H2SO4 Chromic Acid Test o Exactly the same as Oxidation Reactions o Positive Test (+) = 1 /2 Alcohol (color change: red/orange blue/green) o Negative Test (-) = 3 Alcohol K2Cr2O7/H2SO4 (Visible Change: orange/red blue/green) 1 Alcohol Aldehyde + H2O K2Cr2O7/H2SO4 (Visible Change: orange/red blue/green) 2 Alcohol Ketone + H2O K2Cr2O7/H2SO4 3 Alcohol NR
Lucas Test o Substitution Reactions o Rate of Reaction is Key Formation of ppt 3 Alcohol = Fast < 1 min 2 Alcohol = Slow < 5-15 min 1 Alcohol = NR or > 30 min [ZnCl2] Alcohol + HCl Alkyl Chloride (s) + H2O (Note the speed of formation) Examples:
You Try It: Diagnostic Tests
Summary 14 Reaction 3 Reactions Reaction Type Recognize Mechanism Misc Memorize Gain Bond O [O] Chromic Acid Test Oxidation [-H2O] Find the Water Dehydration 2 Reactants (not Oxid or Dehyd) Na loves Cl Lucas Test Swap Substitution
Summary 14 Reaction 3 Reactions