Reactions of Carbonyl Compounds: Introduction and Examples
Discover the key reactions of carbonyl compounds, including addition reactions and reduction processes. Explore the formation of hemiacetals, addition of alcohols, and reactions with hydrogen cyanide and amines. Examples and illustrations provided for better understanding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
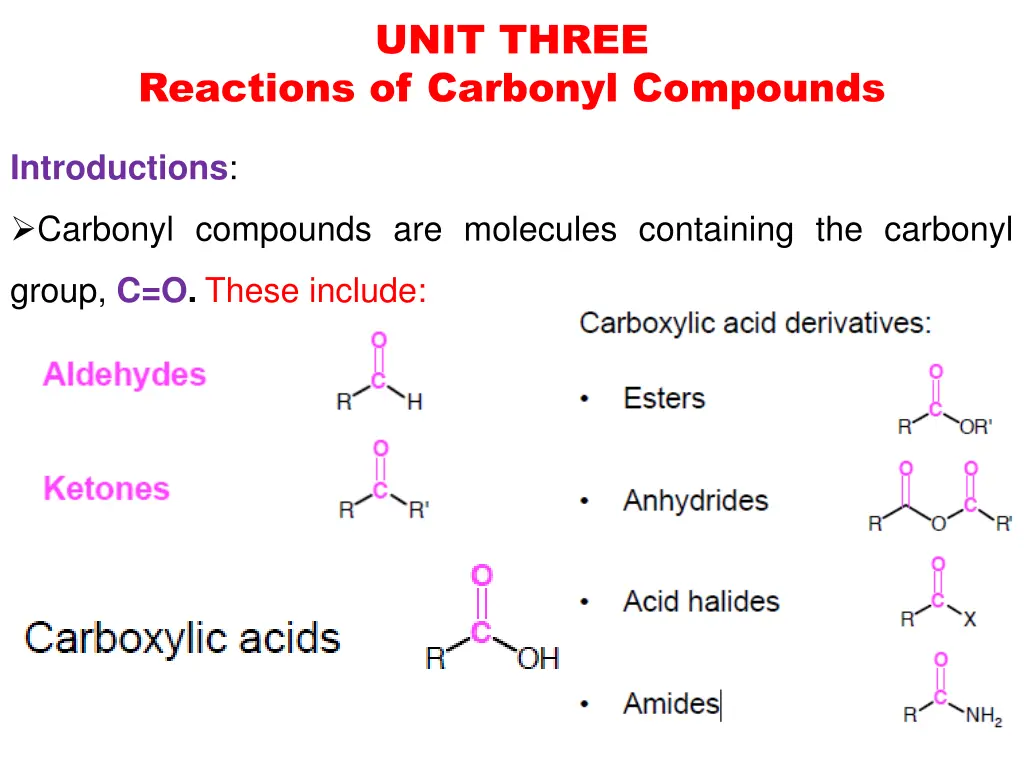
UNIT THREE Reactions of Carbonyl Compounds Introductions: Carbonyl compounds are molecules containing the carbonyl group, C=O.These include:
Rxn of C=O There are some useful reactions of carbonyl compounds involve carbon hydrogen bonds adjacent to the carbonyl group. Such reactions, which can be regarded as the backbone of much synthetic organic chemistry, This is usually result in the replacement of the hydrogen by some other atom or group, For instance:
Addition Reactions Hemiacetal Formation i i) Hydration and ) Hydration and Hemiacetal Formation Water adds rapidly to the carbonyl function of RCHO and R2CO In most cases the resulting hydrate (a geminal-diol) is unstable relative to the reactants and cannot be isolated. There is an Exceptions to this reaction, one being formaldehyde (a gas in its pure monomeric state). Thus, a solution of formaldehyde in water (formalin) is almost exclusively the hydrate, or polymers of the hydrate.
ii) Addition of Alcohols ii) Addition of Alcohols
Examples (1) Examples (1)
Examples (2) Examples (2)
iii) Addition of hydrogen cyanide to C=O iii) Addition of hydrogen cyanide to C=O
iv) The reduction of an RCHO and R iv) The reduction of an RCHO and R2 2CO CO Using lithium tetrahydridoaluminate or sodium, tetrahydridoborate, same organic product yield. For example,
v) Reactions of C=O with Amines Aldehydes and ketones react with primary amines to form imines, or Schiff bases. The mechanism of imine formation involves the nucleophilic addition of the amine to the carbonyl carbon, It forms a stable intermediate species called a carbinolamine.
Contd, Cont d , Carbinolamines are compunds with an amine group and a hydroxy group attached to the same carbon; Are analogous to hemiacetals. Mechanism of Carbinolamine Formation Carbinolamine formation begins with nucleophilic attack on the carbonyl carbon, The product of this attack is a neutral, charge-separated species, Water and its conjugate acid both play roles in the reaction.
Reaction of C=O With Grignard Reagents For instance: the reaction between Grignard reagents and methanal;
The reaction between Grignard reagents and other aldehydes The reaction between Grignard reagents and ketones
Addition-elimination Reactions of C=O A) Formation of Imines and Related Compounds A) Formation of Imines and Related Compounds The reaction of aldehydes and ketones with ammonia or 1 -amines forms imine derivatives, also known as Schiff bases, (compounds having a C=N function).
B) Wittig Reaction B) Wittig Reaction Phosphorus ylides are easily prepared from triphenyl phosphine and primary or secondary alkyl halides. Their preparation involves two reactions;
Reaction II, Acetals Acetals are functional groups characterized by an sp3-C that is bonded to two oxygen atoms; which are themselves attached to a carbon atom.
Formation of Acetals sometimes also known as ketals, are readily formed from one molecule of an aldehyde or ketone and two molecules of an alcohol under acidic conditions
Mechanism of Acetal Formation Under acidic conditions, R-OH becomes protonated ROH2+. The hemiacetal OH oxygen abstracts a proton from ROH2+. Loss of H2O gives a resonance-stabilized alkoxy carbocation. Nu- attack by the alcohol on the carbocation occurs. Deprotonation by a further alcohol molecule produces the acetal
Ester hydrolysis Ester hydrolysis Hydrolysis is a chemical process in which a certain molecule is split into two parts by the addition of a molecule of water. One fragment of the parent molecule gains a hydrogen ion, other group collects the remaining hydroxyl group. Ester is hydrolyzed when treated with excess of water. This reaction is reverse of ester synthesis from corresponding carboxylic acid and alcohol. Reaction is catalyzed by acid or base.
Contd, Both yield same product, except that, in base catalyzed reaction salt of carboxylic acid is obtained from which acid can be regenerated by acidic. Acid catalyzed reaction is reversible; get product in good yields, it is necessary to use dilute acid and ample amount of water. Mechanism of ester hydrolysis (AAC1 mechanism ) i) Acid catalyzed, unimolecular, acyl oxygen fission
For example Hydrolysis of methyl mesitoate ii) Acid catalyzed, bimolecular, acyl oxygen fission;
iii) Acid catalyzed, unimolecular, alkyl oxygen fission iv) Acid catalyzed, bimolecular, alkyl oxygen fission
A) Base catalyzed, unimolecular, acyl oxygen fission B) Base catalyzed, bimolecular, acyl oxygen fission
C) Base catalyzed, unimolecular, alkyl oxygen fission D) Base catalyzed, bimolecular, alkyl oxygen fission
Acid Chlorides The functional group of an acid halide is an acyl group bonded to a halogen. The most common are the acid chlorides. To name, change the suffix -ic acid to -yl halide O O O O Cl Cl Cl RC- CH3CCl O An acyl group Benzoyl chloride Ethanoyl chloride (Acetyl chloride) Hexanedioyl chloride (Adipoyl chloride) Acid chlorides are prepared from carboxylic acids using either thionyl chloride (SOCl2) or phosphorus penta-chloride (PCl5)
Reactions of Acid chlorides, Reactions of Acid chlorides, 1. Conversion into acids and derivatives: a) hydrolysis b) ammonolysis c) alcoholysis 2. Friedel-Crafts acylation 3. Coupling with lithium dialkylcopper 4. Reduction
1) conversion into acids and other derivatives 1) conversion into acids and other derivatives O O H2O Hydrolysis Cl OH isovaleryl chloride 3-methylbutanoyl chloride isovaleric acid 3-methylbutanoic acid O O NH3 Ammonolysis CH3CH2 C CH3CH2 C Cl NH2 propionyl chloride propanoyl chloride propionamide propanamide CH3CH2OH O O Alcoholysis C C OCH2CH3 Cl benzoyl chloride ethyl benzoate
Schotten-Baumann technique Ar- acid chlorides are less reactive than aliphatic acid chlorides. In order to speed up the reactions of aromatic acid chlorides, bases such as NaOH or pyridine are often added to the reaction mixture. O2N O2N CH3CH2CH2OH O C COCl pyridine OCH2CH2CH3 O2N O2N n-propyl-3,5-dinitrobenzoate 3,5-dinitrobenzoyl chloride
2) 2) Friedel Friedel- -Crafts Crafts acylation acylation O AlCl3 O + HCl R C Ar + ArH R C Cl phenone O O AlCl3 + CH3 CH3CH2CH2C Cl CH3CH2CH2C CH3 + ortho- butyryl chloride toluene p-methylbutyrophenone O AlCl3 + CH3CH2CH2C Cl No reacton NO2 butyryl chloride
3) coupling with lithium 3) coupling with lithium dialkylcopper O dialkylcopper O + R'2CuLi R C R' R C Cl ketone O O C CH2CH2CH3 + (CH3CH2CH2)2CuLi C Cl butyrophenone lithium di-n-propylcopper benzoyl chloride O O + C 2CuLi Cl isobutyryl chloride lithium diisopropylcopper 2,4-dimethyl-3-pentanone
4) Reduction to 4) Reduction to aldehydes aldehydes O LiAlH(t-BuO)3 O R C R C Cl H O O LiAlH(t-BuO)3 C C Cl H mechanism, nucleophilic acyl substitution by hydride :H- O O 1) + :H RDS R C R C Cl H Cl O O + Cl 2) R C R C Cl H H
Acid anhydrides The functional group of an acid anhydride is two acyl groups bonded to an oxygen atom. The anhydride may be symmetrical (two identical acyl groups) or mixed (two different acyl groups). To name, replace acid of the parent acid by anhydride. O O O O COC CH3COCCH3 Acetic anhydride Benzoic anhydride Cyclic anhydrides are named from the dicarboxylic acids from which they are derived.
For instance: O O O O O O O O O Succinic anhydride Phthalic anhydride Maleic anhydride Acid anhydrides may be prepared from the condensation product of two carboxylic acids, with loss of water. Anhydrides are formed by treatment of carboxylic acids with strong dehydrating reagents.
Contd, Cyclic anhydrides may be formed by treating a dicarboxylic acid with another anhydride. For example: Reactions of Anhydrides, Reactions of Anhydrides, 1) Conversion into carboxylic acids and derivatives. a) hydrolysis b) ammonolysis c) alcoholysis 2) Friedel-Crafts acylation
Conversion into carboxylic acids and Conversion into carboxylic acids and derivatives derivatives O COOH + H2O O COOH O phthalic acid phthalic anhydride O O + + NH3 CH3 C (CH3CO)2O CH3 C NH2 ONH4 acetic anhydride acetamide ammonium acetate O O CH2COCH2CH3 CH2COH O + CH3CH2OH O O ethyl hydrogen succinate succinic anhydride
Friedel Friedel- -Crafts Crafts acylation acylation O O AlCl3 (RCO)2O + ArH R C Ar phenone + R C OH O AlCl3 CH3 H3C C CH3+ CH3CO2H + (CH3CO)2O acetic anhydride toluene p-methylacetophenone O O AlCl3 + O C O C OH O phthalic anhydride o-benzoylbenzoic acid
Amides The functional group of an amide is an acyl group bonded to a nitrogen atom; drop -oic acid from the name of the parent acid and add - amide. (For the common acid name, drop -ic of the acid name and add -amide.) an alkyl or aryl group bonded to the N: name the group and show its location on nitrogen by N-. O O O CH3 H H-C-N CH3CNH2 CH3C-N CH3 CH3 N-Methylacetamide (a 2 amide) N,N-Dimethyl- formamide (DMF) (a 3 amide) Acetamide (a 1 amide)
Chemistry of Amides Amides are usually prepared by reaction of an acid chloride with an amine. Ammonia, monosubstituted and disubstituted amines (but not trisubstituted amines) all react. O .. NR3 no reaction NH3 O R C Cl 3 amine R C NH2 NHR2 2 amine NH2R 1 amine 1 amide O O R C NR2 R C NHR 3 amide 2 amide
Reaction of Amides i i) ) Alcoholysis Alcoholysis of Amides of Amides Alcoholysis of amides occurs by the same acid catalyzed mechanism as acid hydrolysis except that the amido group of the amide is replaced with by an alcohol rather than water. Dry acid like HCl(g) or H2SO4 must be used; otherwise water would compete with the alcohol as the nucleophile producing some carboxylic acid product in place of an ester. The reaction will require a long reflux period because amides are weak electrophiles and alcohols are weak nucleophiles
Possible Illustration 1 O OH O + CH3CHCH2CH3 C NH2(CH3)2 + C N(CH3)2 O H2SO4 CH3CHCH2CH3 N,N-dimethylcyclopentanecarboxamide sec-butyl cyclopentanecarboxylate ii) Hydride Reduction of Amides ii) Hydride Reduction of Amides Amides are reduced by LiAlH4 and its product is an amine rather than an alcohol. The amide carbonyl group is converted to a methylene group (- C=O -CH2). This is unusual
This unusual; iii) Grignard Reduction of Amides iii) Grignard Reduction of Amides Grignards deprotonate 1 and 2 amides and are not reactive enough to add to the imide ion product. N-H protons are acidic enough (pKa = 17) to be abstracted by Grignards. .. _ : : : : O O :CH3+MgBr .. .. H R R R C N C N _ :CH3+MgBr R CH4 imide anion 2 amide
iv) Acidic Reaction with H2O - Amides Hydrolysis of an amide in aqueous acid requires a mole of acid per mole of amide. Reaction is driven to completion by the acid-base reaction between the amine or ammonia and the acid O O H2O + Cl- H2O HCl NH4 + OH + + NH2 heat Ph Ph 2-Phenylbutanamide 2-Phenylbutanoic acid Mechanism: Acidic H2O - Amides Step1: Protonation of the carbonyl oxygen gives a resonance- stabilized cation intermediate;
Mechanism: Acidic H2O - Amides O + R C NH2 + H O H + H H H H O C NH2 O O C + + R NH2 + R R C NH2 H2O Resonance-stabilized cation intermediate Step 2: Addition of water (a nucleophile) to the carbonyl carbon (an electrophile) followed by proton transfer gives a TCAI. proton transfer from O to N OH OH OH + + + R C O NH3 R C NH2 O H H R C O NH2 + H H H
Step 3: Collapse of the TCAI and proton transfer. (Elimination H O + O H O + + NH3+ + NH3 R C OH NH4 R C OH R C OH v) Basic Reaction with H2O Amides Hydrolysis of an amide in aqueous base requires a mole of base per mole of amide and its rxn is driven to completion by the irreversible formation of the carboxylate salt. O O H2O heat CH3CO-Na+ + CH3CNH H2N + NaOH N-Phenylethanamide (N-Phenylacetamide, Acetanilide) Sodium acetate Aniline
Reductions of acid derivative i) Reduction to Alcohols LiAlH4 reduces acids, acid chlorides, and esters to primary alcohols. ii) Reduction to Aldehydes Acid chlorides will react with a weaker reducing agent to yield an aldehyde.
iii) Reduction to Amines LiAlH4 reduces amides and nitriles to amines. Nitriles and 1 amides reduce to 1 amines. 2 amide reduces to a 2 amine. 3 amide reduces to a 3 amine. Enols and Enolates