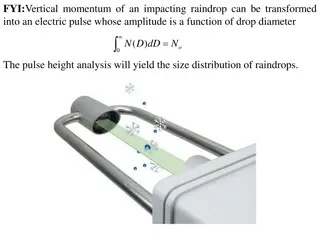
RISE Proposal: Physics Research at Intensity Frontier
"This RISE proposal focuses on cutting-edge physics research at the intensity frontier, involving flavor physics, neutrino oscillation physics, detector construction, and more. The project aims to merge different research areas to tackle common problems and leverage Japan's unique infrastructure. Excellence is emphasized with innovative approaches and multidisciplinary collaboration, while the impact includes providing new career perspectives and advancing fundamental discovery tools."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Heterocyclic Organic Chemistry CHEM 341 Dr. Sultan Almadhhi Assistant Professor of Organic Chemistry King Saud University
o Cyclic organic compounds are carbocycles or heterocycles Carbocycle rings contain only carbon atoms Heterocycle rings atoms in addition to carbon (N,S,O are common) o Heterocycles include many important natural materials as well as pharmaceuticals. o Heterocyclic systems are important building-blocks for new materials possessing interesting electronic, mechanical or biological properties. 3
Some Drugs Containing Heterocycles Initially studied for use in hypertension (high blood pressure) and angina pectoris, but that it could induce marked penile erections. 4
Classification of Heterocycles A. Heterocyclic compounds can be classified into three types based on their general structural; 1. Saturated systems Heterocycloalkanes 2. Partially Unsaturated systems Heterocycloalkenes 3. Aromatic systems - Heteroannulenes B. Heterocyclic compounds can be classified into two types based on the variety of structure; 1. Five membered heterocyclic compounds 2. Six membered heterocyclic compounds 3. Fused or condensed heterocyclic compounds 5
Classification of Heterocycles o Heterocyclic compounds can be classified into three types based on their general structural; 1. Saturated heterocyclic compounds 2. Partially unsaturated heterocyclic compounds 3. Aromatic heterocyclic compounds 6
Classification of Heterocycles 1. Saturated Heterocyclic Compounds o Saturated heterocyclic compounds are heterocycloalkanes include cyclic amines, cyclic amides, cyclic ethers, and cyclic thioethers. o Saturated heterocyclic compounds without double bonds. o Examples; 7
Classification of Heterocycles 2. Partially Unsaturated Heterocyclic Compounds o Partially Unsaturated heterocyclic compounds are heterocycloalkenes o Partially Unsaturated heterocyclic compounds with one or tow double bonds. o Examples; 8
Classification of Heterocycles 3. Aromatic Heterocyclic Compounds o Aromatic heterocyclic compounds are similar to benzene. o To be classified as aromatic, a compound must have: 1) Cyclic structure 2) Cyclic structure contains what looks like a conjugated system of alternating double and single bonds 3) Aromatic compounds must be planar 4) Fulfill Huckel rule The number of electrons in the compound = 4n + 2 Where (n = 0,1, 2, 3, and so on) 9 = 6 6 6 10 6 10
Classification of Heterocycles 3. Aromatic Heterocyclic Compounds o Examples; = 6 = 4 n = 0.5 = 12 n = 2.5 = 8 n = 1.5 n = 1 Not Not Not Not Aromatic Aromatic Aromatic Aromatic 10
Classification of Heterocycles o Aromatic heterocyclic compounds can be classified into three types; i. Aromatic Five membered heterocyclic compounds ii. Aromatic Six membered heterocyclic compounds iii. Aromatic Fused heterocyclic compounds 11
Nomenclature 12
Nomenclature o Many heterocyclic compounds are known by their common names, which makes it difficult for chemists to remember all these names. This has led to the necessity of a systematic method for naming these compounds. o The International Union of Pure and Applied Chemistry (IUPAC) has adopted method for naming such compounds, which allow three nomenclatures; 1. Common names Trivialnames 2. The Hantzsch-Widman nomenclature 3. The Replacement Nomenclature 13
1. Common names Trivial names o There are many heterocyclic compounds widely known by their non-systematic or common names. These names are recognized in scientific circles without a systematic basis, derived from ancient languages like Latin to indicate compound properties such as usage or smell. They are frequently used for their simplicity and can be combined with other naming methods, as they do not follow a scientific rule and are memorized. o In this course, will be focused on compounds that contain Oxygen O , Sulfur S , and Nitrogen N atoms as heteroatoms due to their significance. 14
1. Common names Trivial names o Examples; Saturated heterocycles Six-membered heterocycles Five-membered heterocycles 15
1. Common names Trivial names 16 Fused heterocycles
2. The Hantzsch-Widman Nomenclature o The Hatsch-Wiedemann method is used for naming heterocyclic compounds, whether they are saturated or unsaturated. This naming system includes a prefix at the beginning of the name to indicate the type of heteroatom, stem at the middle to indicate the ring size, and suffix at the end to indicate the degree of saturation. If applicable, the locants number is placed at the beginning of the name (before the prefix). Locants Prefix Stem Suffix Degree of Saturation Heteroatom If Applicable Ring Size [Table 3] [Table 1] [Table 2] 17
2. The Hantzsch-Widman Nomenclature o Before naming the compound, it is essential to identify three main aspects; a. What type of heteroatom Z is present in the compound? b. What is the ring size n ? c. Does the compound contain a Nitrogen atom or not? And is the compound saturated or not? n= 1, 2, 3, .. 18
2. The Hantzsch-Widman Nomenclature a. The type of heteroatom is indicated by a prefix according to Table 1 below; Heteroatom Prefix O Oxa- N Aza- S Thia- b. The ring size is indicated by a stem according to Table 2 below; Ring Size Latin Numerals Ring Size Latin Numerals Stem Stem 3 tri -ir- 7 hepta -ep- 4 tetra -et- 8 octa -oc- 5 - -ol- 9 nona -on- 6 - -in- 10 deca -ec- 19
2. The Hantzsch-Widman Nomenclature c. The suffix to indicate the Nitrogen containing and degree of saturation according to Table 3 below; Suffix Ring with Nitrogen Ring without Nitrogen Ring Size Stem Unsaturated Saturated Unsaturated Saturated 3 -ir- -ine -idine -ene -ane 4 -et- -e -idine -e -ane 5 -ol- -e -idine -e -ane 6 -in- -e Perhydro- - -ane 7 -ep- -ine Perhydro- -in -ane 8 -oc- -ine Perhydro- -in -ane 9 -on- -ine Perhydro- -in -ane 10 o Unsaturated; the ring contains the largest possible number of -ec- -ine Perhydro- -in -ane 20 bonds.
2. The Hantzsch-Widman Nomenclature o The suffix for partially unsaturated heterocyclic compounds, according to Table 4 below; Suffix Ring with Nitrogen Ring without Nitrogen Ring Size Stem Partially Unsaturated 4 -et- -ine -ene 5 -ol- -ine -ene o According to this system heterocycles are named by combining appropriate prefix, stem,and suffix,the letter a in the prefix is omitted where necessary. 21
2. The Hantzsch-Widman Nomenclature I. Monocyclic Heterocyclic Compounds: o Rules: 1) The heteroatom is always assigned the number one. Aza + ol + idine Azolidine Aza + ol + e Aza + in + Perhydro Aza + in + e Azole Perhydroazine Azine 22 Thia + oc + ane Thiaocane Oxa + ep + in Oxepin
2. The Hantzsch-Widman Nomenclature I. Monocyclic Heterocyclic Compounds: 2) If another substituent is present, the heteroatom should be given the lowest possible numbering. Aza + ol + e 3-Chloro-1H-azole Oxa + ir + ane Ethyloxirane 23
2. The Hantzsch-Widman Nomenclature I. Monocyclic Heterocyclic Compounds: 3) If the compound is partial unsaturation, the name of unsaturated will be used with dihydro, tetrahydro, etc. Thia + ol + ene 2,5-Dihydrothiaolene Aza + ep + ine 2,3-Dihydro-1H-azepine 2,5,6-Tetrahyrooxocine Oxa + oc + in 24
2. The Hantzsch-Widman Nomenclature I. Monocyclic Heterocyclic Compounds: 4) Prioritize numbering for saturated atoms Aza + ep + in Oxa + oc + in 1,4-Dimethyl-4,5-dihydroazepin 3,5-Dimethyl-2,4,8-Tetrahydrooxocin 25
2. The Hantzsch-Widman Nomenclature I. Monocyclic Heterocyclic Compounds: 5) When multiple heteroatoms are present, the preferred order is Oxygen O first, followed by Sulfur S , and then Nitrogen N . [Thia + Aza] + ol + e 1,3-Thiazole [Oxa + Aza] + ol + e 1,2-Oxazole [Oxa + Aza] + ir + idine 26 Oxaziridine
2. The Hantzsch-Widman Nomenclature I. Monocyclic Heterocyclic Compounds: 6) When two or more similar atoms contained in a ring are indicated by the prefixes di- , tri- , and etc. Aza + ol + e 1H-1,2,3-Triazole Aza + ol + e 1H-1,2-Diazole Aza + ol + e 1H-1,2,4-Triazole Aza + in + e 1,3,5-Triazine 27
2. The Hantzsch-Widman Nomenclature I. Monocyclic Heterocyclic Compounds: 7) The ring is numbered to give the smallest possible number to other heteroatoms, with substituent position not affecting the numbering. [Thia +Aza] + ol + e 4-methyl-1,3-thiazole [Oxa +Aza] + ol + e 4,5-Dimethyl-1,3-oxazole 28
2. The Hantzsch-Widman Nomenclature I. Monocyclic Heterocyclic Compounds: 8) If different alkyl substituents are attached on the ring, they are named in order of alphabetical order. Thia + ol + e Oxa + ol + ane 3-Bromo-4-methyloxolane 3-Ethyl-2-methylthiazole 29
2. The Hantzsch-Widman Nomenclature I. Monocyclic Heterocyclic Compounds: o Examples: 4-Nitro-azine 2-Amino- 1,3-diazine 2H-Oxin 4H-Thiain 30
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: o Fusion: This term refers to the process of joining two separate rings at two atoms and one common bond, ensuring the maximum number of non-cumulative double bonds. o Ortho-fused rings: These rings share exactly two common atoms and one bond. i.e., Naphthalene. 31
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: o Ortho- and peri-fused rings: These are polycyclic compounds where one ring is ortho-fused to two other ortho-fused rings, sharing three common atoms. An example is 1H-phenalene, composed of three ortho-peri-fused benzene rings. o Polycyclic compounds that include a heterocyclic ring or a fused heterocyclic system attached to a benzene ring are called benzoheterocycles. o Bicyclic compounds with two fused heterocyclic rings are also common and can be named according to specific rules. 32
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 1. Nomenclature of benzo fused compounds: o If a benzene ring is fused to a heteromonocycle with five or six members or to a heterobicycle (base), and it is not listed as a trivially named heterobicycle (See slide 15). o The name of the fused ring is attached as a prefix and the name has the ending o . i.e., Benzo. o When naming such compounds the side of the heterocyclic ring is labeled by the letters a, b, c, etc. starting from the atom numbered 1. Therefore side a being between atoms 1 and 2, side b between atoms 2 and 3, and so on. Benzo + [letter] + Name of base ring 33
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: Benzo[b]furan Benzo[c]thiophene Benzo[b]pyrrole Indole Benzo [b] pyridine Qunioline 34
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: o For compounds where a benzene ring is fused to a heterocyclic ring without a common name, the name is formed by using 'benzo' as a prefix, followed by the heterocyclic symmetrical name, with numbers indicating the positions of the heteroatoms. Benzo [d] thiepin 35
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 2. Nomenclature of fused heterocyclic compounds: o The name of the heterocyclic ring is taken as the parent compound (base) and the ring containing Nitrogen N is given priority, followed by Oxygen O and then Sulfur S . o To name fused heterocyclic systems consisting of two monoheterocyclic units or benzoheterocycles (e.g., chromene) fused with another heterocyclic ring, one system is regarded as the parent (base), while the other is considered a substituent. Fused [number, number - letter] Base Name of fused ring + [number, number - letter] + Name of base ring 36
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 2. Nomenclature of fused heterocyclic compounds: o The name of the fused ring is formed by using a contracted prefix for the substituent ring, where the terminal 'e' in the prefix is changed to 'o,' with certain exceptions: Heterocyclic compound as Prefix Furan Imidazole Thiophene Pyridine Pyrimidine Quinoline Isoquinoline Prefix Furo Imidazo Thieno pyrido Pyrimido Quino Isoquino 37
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 2. Nomenclature of fused heterocyclic compounds: o The numbers indicate the atoms in the fused ring that are shared with the base ring. o The sequence of the numbers shows which atom of the fused ring is encountered nearest to atom 1 in the major ring numbering system (these numbers may be listed in ascending or descending order, such as 2,3 or 3,2). o The letter specifies the attachment points where the fused ring connects to the base ring (fusion sites within the base component). o The suffix indicate the name of the base ring is written, and prefix the name of fused ring. o The fused system has three numbering systems: one for the 38 fused ring, one for the base ring, and one for the whole system.
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: o Priority order of ring systems: The selection of a base or Fused ring follows these rules in the given sequence: 1) As mention before, the name of the heterocyclic ring is taken as the parent compound (base) and the ring containing Nitrogen N is given priority, followed by Oxygen O and then Sulfur S . Base ring because has N Pyrrole Fused ring Chromene Chromeno [2,3-c] pyrrole 39
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: Base ring O preferred to S Fused ring 7H-Thiopyrano [3,2-b] furan Base ring N preferred to O Fused ring 6H-Furo [2,3-b] pyrrole 40
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 2) If the heteroatoms are the same in two different ring sizes, the base ring will be the largest membered ring and the other is fused ring. 2H-Furo [3,2-b] pyran Base ring 2H-pyan Fused ring 2,5-dihydrofuran 41
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 3) The ring with greater number of heteroatoms of any kind will be the base ring. 5H-Pyrido [2,3-d] [1,2]oxazine Fused ring Pyridine Base ring 6H-1,2-oxazine 42
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 4) If the same number of heteroatoms where in the two rings, the base ring will have a greater variety of heteroatoms. Base ring Oxazole 1H-Pyrazolo [4,3-d] [1,3]oxazole Fused ring 1H-pyrazole 43 Base ring Thiazole 1H-Imidazo [4,5-d] [1,3]thazole Fused ring 1H-imidazole
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 5) If the two rings have variable heteroatoms, the base ring will have the most preferred heteroatom according to the following number; 44 Base ring Fused ring Oxazole [1,3] Thiazolo [5,4-d] [1,3]oxazole Thiazole
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 6) If the two rings have the same number of heteroatoms, the base ring will have heteroatom with lower locants. Pyrazino [2,3-d] pyridazine Fused ring Pyrazine Base ring Pyridazine 45
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 7) If a heteroatom occupies a position of fusion, the base ring will have a greater variety of heteroatoms. Imidazo [2,1-b] [1,3]thiazole Fused ring 1H-imidazole Base ring [1,3]thiazole 46
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: o Number the fused system starting from the first atom after the fusion point, following the rules in sequence: 1) The heteroatoms saturated will have the lowest possible numbers. NOT NOT 1H-furo [2,3-d] imidazole 2) The heteroatoms of higher priority will have the lowest numbers. NOT 47 4,5-dihydrothieno [2,3-d] furan
2. The Hantzsch-Widman Nomenclature II. Fused Heterocyclic Compounds: 3) The fusion carbon atoms will have the lowest possible numbers. NOT NOT Imidazo [1,2-b][1,2,4]triazine imidazole 4) The indicated hydrogen atoms will have the lowest numbers. 48 NOT 4H-dioxolo [4,5-d] imidazole
2. The Hantzsch-Widman Nomenclature III. Identical systems connected by a single bond: o These compounds are classified using prefixes; bi-, tert-, and quater-, based on the number of systems, with the bonding specified accordingly: 2,2 -Bipyridine 2,2 : 4,3 -Terthiophene 49
3. The Replacement Nomenclature o In replacement nomenclature, the name of a heterocycle is derived from the name of the corresponding carbocycle, with a prefix added to indicate the specific heteroatom present in the ring. For example, "aza," "oxa," and "thia" are prefixes used for Nitrogen N , Oxygen O , and Sulfur S atoms, respectively. The numbering of heterocyclic rings is done in a way that assigns the lowest possible number to the heteroatom. 50