RUBY-II Study: Ombitasvir/Paritaprevir/Ritonavir + Dasabuvir for HCV Genotype 1a or 4 with Severe Renal Impairment
Study on non-cirrhotic, treatment-naive patients with stage 4 or 5 chronic kidney disease, including those on dialysis. Results show high SVR12 rates for genotype 1a and 4 with RBV-free regimen. Minimal adverse events observed.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
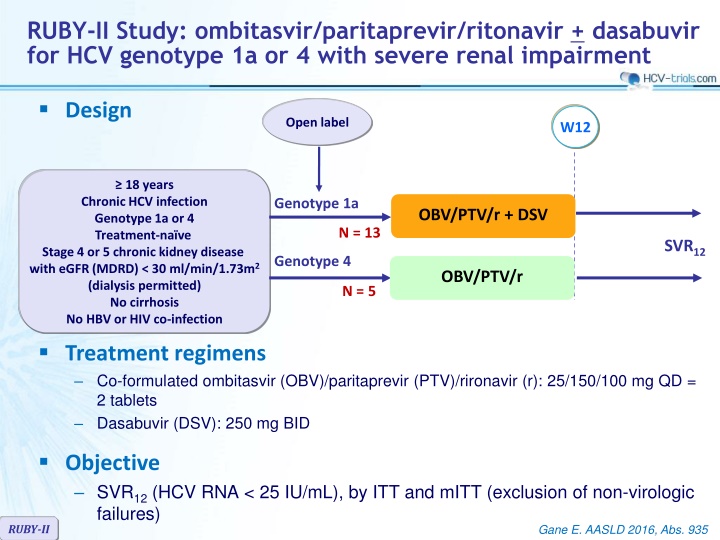
RUBY-II Study: ombitasvir/paritaprevir/ritonavir + dasabuvir for HCV genotype 1a or 4 with severe renal impairment Design Open label W12 18 years Chronic HCV infection Genotype 1a or 4 Treatment-na ve Stage 4 or 5 chronic kidney disease with eGFR (MDRD) < 30 ml/min/1.73m2 (dialysis permitted) No cirrhosis No HBV or HIV co-infection Treatment regimens Co-formulated ombitasvir (OBV)/paritaprevir (PTV)/rironavir (r): 25/150/100 mg QD = 2 tablets Dasabuvir (DSV): 250 mg BID Genotype 1a OBV/PTV/r + DSV N = 13 SVR12 Genotype 4 OBV/PTV/r N = 5 Objective SVR12(HCV RNA < 25 IU/mL), by ITT and mITT (exclusion of non-virologic failures) RUBY-II Gane E. AASLD 2016, Abs. 935
RUBY-II Study: ombitasvir/paritaprevir/ritonavir + dasabuvir for HCV genotype 1a or 4 with severe renal impairment Baseline characteristics and outcome Genotype 1a (N = 13) OBV/PTV/r + DSV 57 31 62 62 / 8 / 31 46 5.8 Genotype 4 (N = 5) OBV/PTV/r 58 40 60 60 / 20 / 20 0 5.7 Median age, years Female, % Race : white, % Fibrosis stage F0-F1 / F2 / F3, % IL28B CC genotype, % HCV RNA log10IU/mL, median Chronic kidney disease, % Stage 4 Stage 5 0 20 100 (hemodialysis : 62, peritoneal dialysis : 38) 6.7 + 3.0 80 (all on hemodialysis) Mean eGFR, ml/min/1.73 m2 SVR12, % ITT mITT 10.1 + 4.6 100 100 80 * 100 * The patient underwent elective renal transplantation and withdrew consent at treatment W2 and did not achieve SVR12 RUBY-II Gane E. AASLD 2016, Abs. 935
RUBY-II Study: ombitasvir/paritaprevir/ritonavir + dasabuvir for HCV genotype 1a or 4 with severe renal impairment Adverse events and laboratory abnormalities, N (%) OBV/PTV/r + DSV + RBV N = 13 (Genotype 1a) OBV/PTV/r + DSV N = 5 (Genotype 4) Serious adverse event (not related to study drugs) Adverse event leading to discontinuation Adverse event in > 15% in any group, % Abdominal pain Fatigue Diarrhea Headache Hypertension Nausea Pruritus Hemoglobin 8-10 g/dL / 6.5-8 g/dL, N Total bilirubin grade 2 (> 1.5 x ULN), N ALT or AST grade 3, N 3 (23%) 1 (8%) * 1 (20%) 1 (20%) ** 31 23 31 23 23 31 15 4 / 0 0 1 0 20 0 0 20 0 20 2 / 0 0 1 * Discontinued study drug (on treatment D77) due to grade 3 ALT, but achieved SVR12 ** Discontinued study drug due to renal failure and transplantation at treatment W2, and did not achieve SVR12 RUBY-II Gane E. AASLD 2016, Abs. 935
RUBY-II Study: ombitasvir/paritaprevir/ritonavir + dasabuvir for HCV genotype 1a or 4 with severe renal impairment Summary In this study of non-cirrhotic, treatment-na ve patients with stage 4 or 5 chronic kidney disease, including those receiving dialysis, the RBV- free regimen of OBV/PTV/r DSV resulted in ITT SVR12rate of 100% for genotype 1a and 80% for genotype 4 (only 5 patients enrolled), and a mITT SVR12rate of 100% for genotypes 1a and 4, with no on- treatment virologic failure or relapse The RBV-free 2-DAA and 3-DAA regimens were generally well tolerated in this patient population Most adverse events were mild to moderate in severity, and there were no serious adverse event deemed related to study drugs These data suggest that RBV may not be necessary in some genotype 1a- or genotype 4-infected patients with severe renal impairment treated with OBV/PTV/r DSV larger trials are needed to confirm the results of this exploratory study RUBY-II Gane E. AASLD 2016, Abs. 935