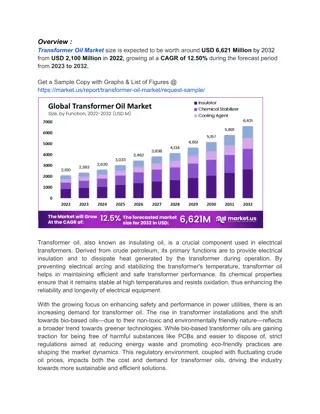
Saponification and Iodine Values in Fats, Oils, and Detergents
Explore the concepts of saponification and iodine values in fats, oils, and detergents as key indicators of chemical properties. Learn about the acid number and its significance in evaluating free fatty acids in oils and fats. Discover the variations in saponification and iodine numbers across different types of oils such as coconut oil, corn oil, olive oil, and more.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Topic :- Topic :- Fats, oils and Detergents Part II Dr. Satish U. Deshmukh Department of Chemistry Deogiri College Aurangabad 1
Fats, oils and Detergents Definition of saponification value: - number of milligrams of KOH required to saponify one gram of a fat or oil, on a molecular basis, one molecule of oil. OR fat requires three molecules of KOH for complete saponification because there are three ester linkages in a fat or an oil molecule. 2
Fats, oils and Detergents Molecular wt. of KOH = 56 g , 3 * 56 = 168 g = 168000 mg Here M g of the fat requires 168,000 mg KOH for saponification. Therefore, one gram of fat or oil will require 168,000/M mg of KOH. Thus, Saponification number = 168000/M Each molecule of a more mol. wt. fat and a low mol. Wt. fat requires three KOH units for saponification. Wt. of KOH required to per gram of fat will be lower for the heavier fat than is required to saponify the low mol. Wt. glycerides. High-molecular weight fats and oil have lower saponification number than oils and fats of low. mol. Wt. Saponification number tells the amount of alkali required by a fat or an oil sample for its conversion to soap. 3
Fats, oils and Detergents The iodine number is a measure of the extent of unsaturation in fats and oils. Definition of iodine value : - It is expressed as the number of grams of iodine that will add to 100 g of the fat or oil being tested. The following equation and calculation illustrate the definition of iodine number. The above equation tells us that 761 4 g of iodine will add to 884 g of fat. The number of grams of iodine that will add to 100 g of oil will be 3 I2= 6 x 126 9 = 761 4g From reaction, 761.4x 100 / 884 mol wt. Of sample = 86 Iodine Number = Amount of iodine consumed by oil or fat in g x 100/mol. Wt of sample in gm 4
Fats, oils and Detergents Fat or Oil Iodine Number Saponification Number 255-258 Coconut Oil 10 Corn Oil 109-133 187-196 Cotton Seed Oil 79-90 187-196 Olive Oil 79-90 187-196 Palm Oil 54 199 Peanut Oil 84-102 188-195 Soybean Oil 127-138 185-195 6
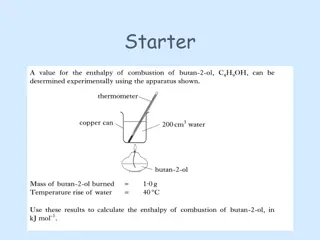
Fats, oils and Detergents Acid Number :- Tell information of free fatty acids present among molecules. The acid number is expressed as the number of mg of KOH required to neutralise 1 gm of oil or fat. Acid number is find out dissolving a wt. total of the fat or oil in ethanol and titrating the solution against standard base. Acid number = 56.1 X a X N/ P Where, a = Vol. of KOH sol. used in ml N = Exact N of the ethanolic KOH solution. P = Wt. of sample in g. 7
Detergents Detergents: Definition :- Chemical substances capable of removing liquid and solid dirt from the surface of a solid. Na and K salts of longer carbon chain fatty acids (COOK) They are insoluble in hard water. 8
Detergents Long Carbon Chain , Non-polar Hydrophobic, Which Is Soluble In Fats, Oils And Greases Detergents Properties Polar. Hydrophilic End Soluble In Water 9
Detergents 10
Detergents Anionic Detergents: Definition :- these are one end water soluble connected to the hydrocarbon chain is negative part alkyl sulphonates Regularly Used Anionic Detergents Alkyl Alkyl-aryl, Sulphonates Alkyl Sulphates, Sulphonates 11
Detergents Synthesis alkyl sulphate:- substituted ethene reacted with sulphuric acid to formed mono alkyl sulphate 12
Detergents Synthesis alkyl sulphate:- substituted ethene reacted with sulphuric acid to formed mono alkyl sulphate 13
Detergents Synthesis alkyl sulphate:- substituted ethene reacted with sulphuric acid to formed mono alkyl sulphate 14
Detergents Teepol is green-coloured detergent. Uses for detergent. Oils ,dirt, and food particle on surface and Reliable NaOH
Sulphonates [1] Sodium Alkyl Sulphonates CL2 SO2 R- H
Sodium Aryl sulphonates Sodium R=alkyl, R=aryl sulphonates are the most commonly produced detergents. They are obtained by alkylation benzene with propylene and sulphonating hydrocarbons then neutralisation with NaOH Sodium Aryl sulphonates are the good detergents and used as household washing powder, laundry washing Sodium Aryl sulphonates 17
Cationic detergents Detergents the more active part are end of is a long-chain cation. Cationic detergents detergents are synthesis of alkyl chloride with fatty amines or tertiary amines. The water-soluble end of the cationic detergent. Cationic detergents is a positive quaternary ammonium salt. Cationic detergents are available in the market in solid foam Or as pastes or in water solution. They are not good detergents and are mainly used as wetting, foaming and emulsifying agents 18
Non-ionic detergents Detergents the more water-soluble end is polar. and can hydrogen bond with water. Due to their union sable characteristic they do not form salts with metallic compounds or with hard water Non-ionic detergents are unique advantages over ionic detergents for use acidic and alkaline solutions. You can prepared to any attractive long chain of polarity. Detergents are bio-degradable. This means that they can be quickly metabolised by microorganisms in a sewage disposal plant and in to released into environment. For a detergent to be biodegradable, the long alkyl chain must be un-branched. Detergents used in early day had branched chains, are not easily biodegradable. Biodegradable detergents are called soft detergents and Non-bio-degradeable detergents are called hard detergents 20
Soaps "Soap making is salts of long-chain fatty acids produced by the saponification of fats and oils known soaps. Metallic salt of a fatty acid is a soap, but the term soap is normally applied to the water soluble salts since only these have detergent properties Sodium salts of a given oil or fat are harder Less soluble than the corresponding potassium salts Saturated fats offers hard soaps Unsaturated fats offers soft soaps. soft soaps are generally the potassium salts, particularly when they are derived from oils. 22
Types of Soaps Soft Soaps 1 Hard Soaps 2 Transparent soap: 3 Metallic Insoluble Soaps 4
Soft soaps Soft soaps are prepared from good oils (coconut) Using base KOH 1 Soft soaps does not contain any types of free alkali metal 2 Soft soaps may add some disinfectants or deodorants 3 Soft soaps are used as body soap, in shaving cream and some shampoo. 4
Hard soaps Hard Soaps are Prepared From Hard Oils 1 Hydrolysis Base NaOH 2 Hard soaps contain any types of free alkali metal 3 Hard soaps are used as for washing purposes 4
Transparent soap Transparent soap prepared by dissolving soft soap in alcohol 1 Transparent soap obtained by getting evaporating the filtrate 2 Transparent soap contain They also contain glycerol. 3
Metallic insoluble soap Soaps of metals other than K and Na insoluble in aqueous layer 1 Not used as cleaning agents. Ca and Mg soaps are used as lubricants 2 Driers purposes Al, Cr, soaps are used in sizing paper, Zn, Fe, soaps for water-proofing of leather and canvas and lead soaps 3 for preparing adhesive.
Metallic insoluble soap Soaps of metals other than K and Na insoluble in aqueous layer 1 Not used as cleaning agents. Ca and Mg soaps are used as lubricants 2 Driers purposes Al, Cr, soaps are used in sizing paper, Zn, Fe, soaps for water-proofing of leather and canvas and lead soaps 3 for preparing adhesive.
Cleansing action of soap 1) Soil particles are held on cloth or on body skin a thin film of fats, oil or grease 2) In order to remove this dirt, the oily material is must first be dissolved 3) So we use water for washing as a solvent for cloth and other material dirt attached 4) But water is a polar liquid [ Two pole ( + and -)] 5) fats and oils, are long hydrocarbon chains, non- polar 6) Hence water insoluble, soaps are structurally capable of solving this more alternatives path to remove the dirt particle.
Cleansing action of soap 7) Soaps are salt of long chain fatty acids. 8) Long alkyl chain having 12 to 18 carbons is non-polar a as the results soluble in fats and oils but insoluble in water other end of soap molecule ( Carbon- carbon chain ) 9) A carboxylic acid salt, is highly polar, in fact it is ionic and is water soluble ( COO-SALT) 10) A soap then has two diverse solubility properties-it has a hydrophilic end (water-loving), soluble in water. and another part is that hydrophobic end (water-fearing), in fats and oils soluble
Cleansing action of soap As a soap dissolves in water, the molecule orient themselves on the water's surface with the ionic end submerged and the non- polar (which is insoluble in water) hydrocarbon chain bobbing above the surface like a sustain top on In this manner Soap molecule satisfies its opposing solubility characteristics the water soluble hydrophilic end is in the water and the hydrophobic end is not in contact with the water. Those soap molecules, which do not find room on the surface of water orient themselves in such a way under the surface, so that the hydrophobic part of the molecules have lesser get in touch with with water. The soap molecule achieve this by grouping in three dimensional clusters, with the non-polar hydrocarbon chains filling the interior of the cluster and the water soluble ionic ends composing the outer surface.
Cleansing action of soap These molecules are gathered together s are called Micelles The solubility characteristics of the soap molecules are satisfied in that all the hydrocarbon chains. are grouped together away from water (a hydrophobic) and the ionic portions are in contact with water. Now if we submerge some soiled clothing in water, the non-polar oil films are loosened and they dissolve in the non- polar hydrocarbon centre of the micelles. Micelles stay colloidal dispersed in the water, with no propensity to coagulate, since there is an ionic repulsion between their charged outer surfaces. The oily films are thus washed away as finely dispersed oil droplets
Fats, oils and Detergents Thank you 35