Scientific Notation, Mole Concept, and Mass Calculations in Chemistry
Explore scientific notation calculations, the concept of atoms and molecules in moles, determining mass by counting units, reasons for mass counting, and the relationships between atomic, formula, and molecular masses in chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
DO NOW Get out Review of Basic Skills packet Pick up notes and get out a calculator. Pick up small piece of paper and put your name on it Look at the jar of M & Ms. Guess how many candies are in the jar. YOU MAY NOT TOUCH THE JAR! Write your guess on the paper and place it in the basket please do NOT fold the paper.
SCIENTIFIC NOTATION 16. Make the following calculations, expressing your answer in scientific notation. a. (8.00 x 1015) (8.00 x 1015) = b. (4.00 x 102) (3.00 x 1012) = c. 8.00 x 1015 2.00 x 106= d. 1.60 x 10-8 2.00 x 1014=
THE MOLE Introduction Matter is made up of particles. A particle can be an atom, ion, molecule, or formula unit. Individual (molecular) atoms Fe He (ionic) Formula units NaCl CaCl2 Molecules H2O N2 ions SO4-2 NH4+1
THE MOLE You should be able to tell how many atoms are in each chemical formula. Fe H2O CaCl2 Mg(OH)2
NUMBER OF CANDIES So, how many candies are there? How can we determine this without actually counting them?
NUMBER OF CANDIES Mass of 10 M&Ms = ____________ Mass of one M&M = ___________ Total mass of M&Ms = ______________ Number of M&Ms in jar = __________
WHY COUNT BY MASSING? So why can t we just count everything? The United States Mint counts money by massing, using well established averages to quickly count by mass millions of coins more accurately than any human or coin counting machine. We certainly cannot count all the atoms in a bottle of water.
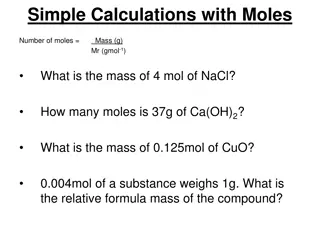
ATOMIC MASS, FORMULA MASS, AND MOLECULAR MASS These are all the same thing, calculated the same way. They are for atoms (atomic mass), ionic compounds (formula mass), and covalent compounds (molecular mass). Answers are always two places past the decimal point since that what all our masses are. Different atoms have different masses; THEREFORE different compounds have masses dependent upon the masses of the atoms that make them up.
ATOMIC MASS This is the mass of an atom in atomic mass units (amu). It is the same as the average mass of the atoms of an element found on the periodic table. Example: C - single atom 12.01 amu Na single atom 22.99 amu Br single atom 79.90 amu
MOLECULAR MASS This is the mass found by adding the atomic masses of the elements found in the molecule (molecular compound). Example: H2O molecular compound H (2 x 1.01amu) = 2.02 O (1x16.00amu) = 16.00 18.02 amu
FORMULA MASS This is mass found by adding up the atomic masses of the elements found in a formula unit (ionic compound). Example: NaCl ionic compound Na (1 x 22.99amu) = 22.99 Cl (1 x 35.45amu) = 35.45 58.44 amu
ATOMIC MASS, FORMULA MASS, AND MOLECULAR MASS Let s try some (show your work): A. H2SO4 C3H5N3O3 B. C. Al(NO3)3 D. Fe(C2H3O2)3
PRACTICE A. H2SO4 H (2 x 1.01 amu) = 2.02 S (1 x 32.06 amu) = 32.06 0 (4 x 16.00 amu) = 64.00 98.08 amu B. C3H5N3O3 C (3 x 12.01 amu) = 36.03 H (5 x 1.01 amu) = 5.05 N (3 x 14.01 amu) = 42.03 O (3 x 16.00 amu) = 48.00 131.11 amu
PRACTICE C. Al(NO3)3 Al (1 x 26.98 amu) = 26.98 N (3 x 14.01 amu) = 42.03 O (9 x 16.00 amu) = 144.00 213.01 amu D. Fe(C2H3O2)3 Fe (1 x 55.85 amu) = 55.85 C (6 x 12.01 amu) = 72.06 H (9 x 1.01 amu) = 9.09 O (6 x 16.00 amu) = 96.00 233.00 amu
PERCENT COMPOSITION We can use these masses to determine the percent by mass. Remember that percent means part divided by whole.
PERCENT COMPOSITION For Example: Chocolate Chip Cookies: 450.0g sugar 450.0g Crisco 2 eggs (75.0g) 900.0g flour Looking at the ingredients, what percentage of the total composition is eggs? 20.0mg salt 20.0mg baking soda 20.0mg vanilla To Calculate: 1. Get the total amount (add up all the ingredients). Total = 450.0g + 450.0g + 75.0g + 900.0g + 0.002g + 0.002g + 0.002g = 1875.006g = 1875.0g 2. Find the percentage of the total that is eggs. 75.0g x 100 = 4.00 % 1875.0 total part x 100
PERCENT COMPOSITION What is the mass percent of hydrogen in sulfuric acid? %H in H2SO4?
TO DO Do the 12 molar masses MY WAY Write the atoms, the numbers, the masses, and do the math. Unit = amu. Calculate the Percent Composition problems on the back. Lab tomorrow need to know your lab partner by then.