Silicones: Polymers with High Temperature Stability
Silicones, also known as poly siloxanes, are organo-silicon polymers with properties like high thermal stability. They can be linear or cross-linked, offering various applications in industries like waterproofing, electrical insulation, and more. Learn about their preparation methods, types such as silicone rubbers and resins, and versatile uses in products like paints, lubricants, and waterproofing clothes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
SILICONES SILICONES SUBMITTED BY ; M.ADITHYA BSC[BZC] ROLL NO:143
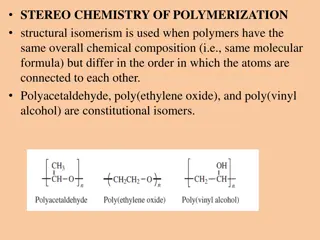
Silcones: Silicones or poly siloxanes are organo silicon polymers with general empirical formula (R2SiO). Since their empirical formula is similar to that of ketone (R2CO), they were named silicones . These silicones may be linear or cross linked. Because of their very high thermal stability they are called high temperature polymers.
Preparation: Generally dialkyldichlorosilanes (R2SiCl2) or diaryldichlorosilanes Ar2SiCl2, which are prepared by passing vapours of RCl or ArCl over silicon at 570 K with copper as a catalyst. silicones are prepared by the hydrolysis of The hydrolysis of dialkylchloro silanes R2SiCl2yields to a straight chain polymer which grown from both the sides
The hydrolysis of monoalkylchloro silanes RSiCl3yields to a very complex cross linked polymer.. Linear silicones can be converted into cyclic or ring silicones when water molecules is removed from the terminal OH groups.
Types of silicones: (i) Liner silicones: They are obtained by the hydrolysis and subsequent condensation of dialkyl or diaryl silicon chlorides. 1. Silicone rubbers: These silicones are bridged together by methylene or similar groups 2. (ii) Cyclic silicones These are obtained by the hydrolysis of R2SiCl2. (iii) Cross linked silicones They are obtained by hydrolysis of RSiCl3 Silicone resins: They are obtained by blending silicones with organic resins such as acrylic esters.
Uses: and in vacuum pumps, high temperature oil baths etc... They are used for making water proofing clothes They are used as insulting material in electrical motor and other appliances They are mixed with paints and enamels to make them resistant towards high temperature, sunlight, dampness and chemicals. Silicones are used for low temperature lubrication
Silicone is identified as a synthetic elastomer as it is a polymer which displays viscoelasticity that is to say it shows both viscosity and elasticity. Colloquially people call these elastic characteristics rubber. Silicone itself is made up of carbon, hydrogen, oxygen and silicon