Silver Compounds Overview
Explore the compounds of silver, including silver nitrate, their properties, reactions, and methods of preparation. Discover how silver nitrate reacts with phosphates, chlorides, sulphides, chromates, thiocyanates, sulphates, and thiosulphates. Learn about the decomposition of silver nitrate and its various applications in chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Compounds of Silver Course Instructor: Dr. Atul Kumar Singh Assistant Professor Department of Chemistry M. L. Arya College, Kasba Purnia -854330 India
Silver Nitrate (Lunar Caustic) Molecular formula: AgNO3 Method of Preparation it is prepared by heating silver with dilute nitric acid
Properties it is a colorless crystalline compounds It is soluble in water and alcohol Melting point : 212 oC It is decomposed by light therefore stored in colored bottles. On heating above 212 oC, it decomposes to silver nitrate and oxygen
When heated at red hot it decomposes to metallic silver . Reaction with Phosphate
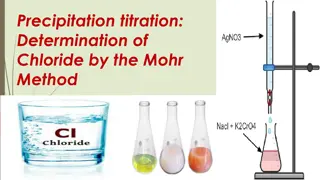
Reaction with Sulphides Reaction with Chromates
Reaction with Thiocyanates . Reaction with Sulphates
Reaction with Thiosulphates White ppt. of Ag2S2O3 which gradually changes to black due to hydrolysis