Simple Bonding Rules for Organic Compounds: HONC Rules & Examples
Explore the simple HONC bonding rules for organic compounds in this informative content. Understand the bond count to lone pairs concept for elements like H, O, N, and C. Dive into examples like ethane, ethanol, aspirin, glycine, and DNA to grasp the practical application of these rules in real compounds. Discover different ways to read and draw HONC structures, from complete skeletal forms to condensed and abbreviated line forms. Enhance your understanding of organic chemical notation and its significance in representing molecular structures accurately.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
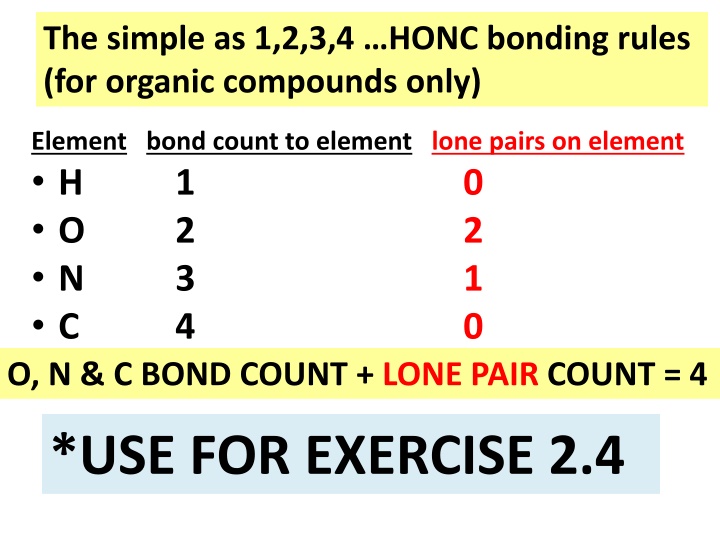
The simple as 1,2,3,4 HONC bonding rules (for organic compounds only) Element bond count to element lone pairs on element H 1 O 2 N 3 C 4 O, N & C BOND COUNT + LONE PAIR COUNT = 4 0 2 1 0 *USE FOR EXERCISE 2.4
Simple Bonding Rules for Organic* compounds: the HONC Rules *Compounds made of C+ H with options to include O and N EXAMPLE #1: ethane (C2H6) = one of the `natural gases H H H C C H H H
EXAMPLE #2: ethanol (C2H6O) =drinking alcohol H H H C C OH H H
EXAMPLE #3: aspirin (C10H12O5 ) = what to take after excess alcohol H OH O C C H H O C H O C C C H C C H C H H C H H H
EXAMPLE #4: glycine (C2H5NO2) =amino acid, building block of proteins (building block of all living things ) H H N H C O H C H O
EXAMPLE #5: DNA (BLUEPRINT for all living things) Deoxyribonucleic acid
POPULAR GRAPHIC FOR DNA
organic chemical notation (see also pp 108-109 of text) 3 different ways to read/draw HONC structures 1) Complete skeletal form H OH H Rubbing alcohol H C C C H H H H All or most all lone pairs, bonds and atoms shown explicitly.
Aside on organic chemical notation (see also pp 108-109 of text) 3 Different ways to read/draw HONC structures (continued) 2) Condensed form OH H OH H H C C C H H3C C CH3 H H H Complete skeletal form H Methyl (CH3) and methylene groups (CH2) written without C-H bonds, but lone pairs still shown.
Aside on organic chemical notation (see also pp 108-109 of text) 3 Different ways to read/draw HONC structures (continued) 3) Abbreviated bond line form (most used by organic chemists) H H OH Complete Skeletal form OH H C C C H H H H All kinks , ends and crossings are C. If no other groups showing,assume H around C to reach 4 bonds to C. Lone pairs assumed via HONC rules(though text doesn t.)
Why bond line form is preferred by organic chemists SIMPLER LOOKING IS PRETTIER OH aspirin O C OH O O O C H H C C C O O C C C H H H C H H Bond line form Complete skeletal form messy,ugly I m neat & pretty
+ more board practice : complete skeletal bond line condensed condensed complete skeletal bond line
and now for the bad news . Ugly chemical fact #1: Lewis Octet rule doesn t always work
EXAMPLE #1: BATTERY ACID (H2SO4) (see also, pg 130 Fig 4.5) O H O O- S H O S OH +2 H O O- O Lewis octet prediction for H2SO4 structure From Experiment (Kuczkowski et. al. 1983) Lone pairs not shown
EXAMPLE #2: Phosphoric acid (H3PO4) EXAMPLE #2: Phosphoric acid (H3PO4) O- OH P+ O O H H H O P OH O H O From experiment Smith et. al. 1954 Lewis octet prediction for H3PO4 structure
EXAMPLE #3: Perchloric acid (HClO4) O O- O- Cl+ +3 H O Cl O O H O- O From experiment Casper et. al. 1994 Lewis octet prediction for HClO4
Exploring the octet vs `experimental structures below (on the board)- what can we learn ?: H2SO4 H3PO4 HClO4
`Old Formal Charge Rule #1 The most stable octet structure also minimizes formal charge (=> zero charge)
Most stable Cl+ Cl Cl Cl C C `excited state molecule of phosgene vs. O- O Mickey Minnie
EXPLAINING DEVIATIONS FROM OCTET RULE: FORMAL CHARGE MINIMIZATION TRUMPS OCTET RULE BEYOND Si `Formal Charge Rule #2 Beyond Si, you can throw away the octet rule to minimize formal charge (=> zero charge)
More examples using formal charge rule 2 SO3, SO2, H3PO3