SMV + PEG-IFN-2a + RBV for HCV Genotype 4 Treatment: Restore Study Overview
Explore the Restore Study on SMV, PEG-IFN-2a, and RBV treatment for HCV genotype 4. The study focuses on achieving SVR12 in patients aged 18-70 with chronic HCV infection, treatment-naïve, or with prior relapse/response issues. Results show promising SVR rates and provide insights on resistance testing outcomes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
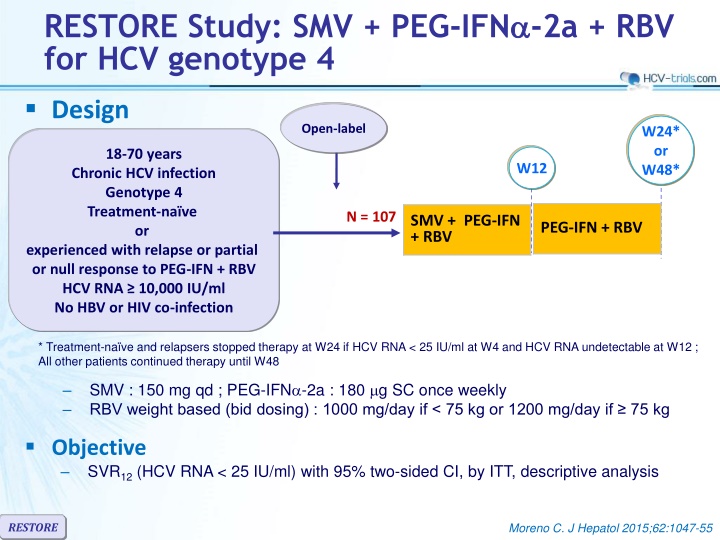
RESTORE Study: SMV + PEG-IFN -2a + RBV for HCV genotype 4 Design Open-label W24* or W48* 18-70 years Chronic HCV infection Genotype 4 Treatment-na ve or experienced with relapse or partial or null response to PEG-IFN + RBV HCV RNA 10,000 IU/ml No HBV or HIV co-infection W12 N = 107 SMV + PEG-IFN + RBV PEG-IFN + RBV * Treatment-na ve and relapsers stopped therapy at W24 if HCV RNA < 25 IU/ml at W4 and HCV RNA undetectable at W12 ; All other patients continued therapy until W48 SMV : 150 mg qd ; PEG-IFN -2a : 180 g SC once weekly RBV weight based (bid dosing) : 1000 mg/day if < 75 kg or 1200 mg/day if 75 kg Objective SVR12(HCV RNA < 25 IU/ml) with 95% two-sided CI, by ITT, descriptive analysis RESTORE Moreno C. J Hepatol 2015;62:1047-55
RESTORE Study: SMV + PEG-IFN -2a + RBV for HCV genotype 4 Baseline characteristics and patient disposition Prior partial Response N = 10 Prior relapse N = 22 Prior null response N = 40 Na ve N = 35 Median age, years 47 52 51 51 Female 26% 14% 0 28% HCV genotype 4a / 4d / other 35% / 24% / 41% 50% / 18% / 32% 30% / 30% / 40% 48% / 25% / 28% IL28B CC genotype 21% 5% 0 0 14% / 36% / 9% / 41% 20% / 30% / 0 / 50% 57% / 17% / 20% / 6% 24% /22% / 16% / 38% Metavir F0-F1 / F2 / F3 / F4 HCV RNA log10IU/ml, median 6.19 5.86 5.84 6.40 Discontinued treatment, N Lost to follow-up Withdrew 2 2 0 0 0 2 1 1 RESTORE Moreno C. J Hepatol 2015;62:1047-55
RESTORE Study: SMV + PEG-IFN -2a + RBV for HCV genotype 4 SVR12(HCV RNA < 25 IU/ml), % (95% CI) % 86.4 100 82.9 ( 70-95) ( 72-100) SVR12in patients with HCV RNA < 25 IU/ml at W4 and undetectable at W12 (RGT) 75 60 (30-90) Prior relapse 40 Na ve (25-55) 50 Met RGT criteria 31/35 20/22 W4 : HCV RNA undetectable 27/28 (96.4%) 17/18 (94.4%) 25 W4 : HCV RNA detectable 2/3 1/1 N 35 22 Prior relapse 10 Prior partial response 40 Prior null response 0 Na ve On-treatment failure, N 3 2 2 18 Virologic breakthrough, N 4 1 2 13 Relapse, N 3 1 2 6 RESTORE Moreno C. J Hepatol 2015;62:1047-55
RESTORE Study: SMV + PEG-IFN -2a + RBV for HCV genotype 4 Resistance testing (NS3) in treatment failure Available in 32/37 28/32 with emergence of NS3 mutations at positions 80, 122, 155, 156 and/or 168 Most frequent profile : D168V alone or D168E mutations at position 80 RESTORE Moreno C. J Hepatol 2015;62:1047-55
RESTORE Study: SMV + PEG-IFN -2a + RBV for HCV genotype 4 Adverse events during the SMV + PEG-IFN + RBV phase, N (%) N = 107 5 (4.7%) 0 1 (0.9%) 6 (5.6%) 1 (0.9%) Serious adverse event Possibly related to SMV AE leading to discontinuation of SMV (valium overdose) Grade 3 adverse events Grade 4 adverse events Most common adverse events Influenza-like illness Asthenia Fatigue Adverse events of clinical interest Anemia Dyspnea Neutropenia Photosensitivity (all grade 1) Pruritus (no grade 3-4) Rash (no grade 3-4) 46% 42% 35% 10% 11% 5% 2% 21% 14% RESTORE Moreno C. J Hepatol 2015;62:1047-55
RESTORE Study: SMV + PEG-IFN -2a + RBV for HCV genotype 4 Summary In this open-label study of genotype 4 infection, 12 weeks of SMV + 24-48 weeks of PEG-IFN + RBV achieved an overall SVR12 of 65% in treatment-na ve and prior relapsers SVR12was 83% and 86%, respectively, which is in line with SVR12rates observed in phase III studies of SMV + PEG-IFN + RBV in patients with genotype 1 Most of na ve and prior relapsers could stop PEG-IFN + RBV at W24 SVR12rates were similar across the different HCV genotype 4 subtypes, with slightly lower rates observed among patients with genotype 4d Adverse events were mainly grade 1 or 2, with few serious adverse events and no deaths Limitations No control arm RESTORE Moreno C. J Hepatol 2015;62:1047-55






















