SMV, PEG-IFN, RBV in Genotype 4 Study
"Explore the open-label study on the efficacy of SMV, PEG-IFN, and RBV treatment in genotype 4 chronic HCV infection for individuals aged 18-70 years. The study focuses on achieving SVR12 with specific treatment protocols and baseline characteristics analysis. Adverse events and treatment discontinuations are also reported. Find out more about the study conducted by Asselah T."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
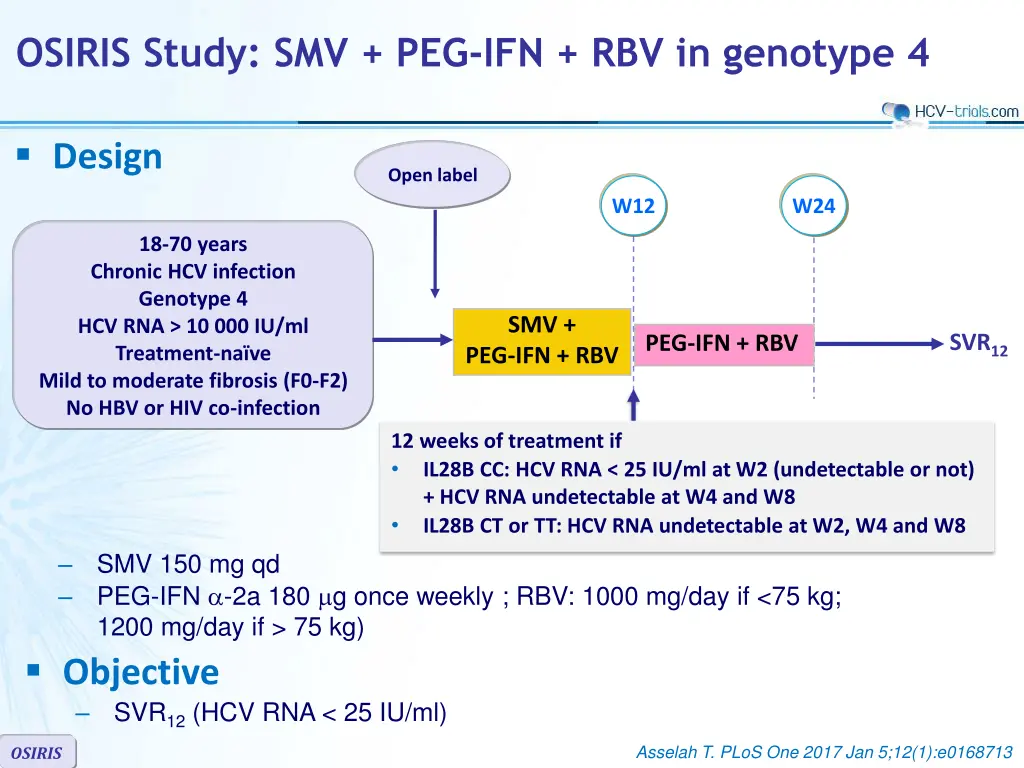
OSIRIS Study: SMV + PEG-IFN + RBV in genotype 4 Design Open label W12 W24 18-70 years Chronic HCV infection Genotype 4 HCV RNA > 10 000 IU/ml Treatment-na ve Mild to moderate fibrosis (F0-F2) No HBV or HIV co-infection SMV + SVR12 PEG-IFN + RBV PEG-IFN + RBV 12 weeks of treatment if IL28B CC: HCV RNA < 25 IU/ml at W2 (undetectable or not) + HCV RNA undetectable at W4 and W8 IL28B CT or TT: HCV RNA undetectable at W2, W4 and W8 SMV 150 mg qd PEG-IFN -2a 180 g once weekly ; RBV: 1000 mg/day if <75 kg; 1200 mg/day if > 75 kg) Objective SVR12 (HCV RNA < 25 IU/ml) Asselah T. PLoS One 2017 Jan 5;12(1):e0168713 OSIRIS
OSIRIS Study: SMV + PEG-IFN + RBV in genotype 4 Baseline characteristics 12 weeks N = 34 48 32 77 / 10 41 24 weeks N = 33 48 30 83 / 14 3 Median age, years Female, % Race : white / black, % IL28B CC genotype, % Genotype subtype, % 4a 4d 4 other HCV RNA, log10IU/ml, median Metavir score, % F0-F1 F2 F3 Discontinued therapy, n (%) SMV (adverse event / virologic endpoint), n PEG-IFN + RBV (adverse event / virologic endpoint), n 41 38 21 5.8 39 36 24 6.4 85 15 0 0 76 21 3 9 (27%) 5 (3 / 2) 9 (4 / 2) Asselah T. PLoS One 2017 Jan 5;12(1):e0168713 OSIRIS
OSIRIS Study: SMV + PEG-IFN + RBV in genotype 4 SVR12by baseline characteristics in the 12W group % 100 100 100 97100 100 100 100 100 97 96 100 94 93 93 92 80 60 40 20 11 14 13 7 29 5 19 15 14 15 5 16 7 23 34 0 Male Female 4a 4d 4/other F0 F1 F3 800K > 800K CC CT TT Europe Middle All East/ Africa Sex HCV subtype Fibrosis stage Baseline HCV RNA (IU/ml) Asselah T. PLoS One 2017 Jan 5;12(1):e0168713 IL28B genotype Region OSIRIS
OSIRIS Study: SMV + PEG-IFN + RBV in genotype 4 SVR12by baseline characteristics in the 24W group % 100 100 88 88 86 86 85 83 82 81 81 80 80 80 75 80 69 60 40 20 23 10 13 12 8 25 7 7 26 1 27 5 16 8 33 0 All Male Female 4a 4d 4/other F0 F1 F3 800K > 800K CC CT TT Europe Middle East/ Africa Region Sex HCV subtype Fibrosis stage Baseline HCV RNA (IU/ml) IL28B genotype Asselah T. PLoS One 2017 Jan 5;12(1):e0168713 OSIRIS
OSIRIS Study: SMV + PEG-IFN + RBV in genotype 4 Adverse events, % 12 weeks, N = 34 24 weeks, N = 33 Serious adverse event Adverse event possibly related to SMV Adverse event possibly related to PEG-IFN Adverse event possibly related to RBV AE leading to discontinuation of all study drugs Grade 3-4 adverse events Adverse events in 20% in either group Pruritus Neutropenia Asthenia Fatigue Decreased appetite Influenza-like illness Headache 0 6 45 79 58 9 42 29 59 50 0 18 26 26 15 18 18 9 15 21 21 27 24 24 27 24 2 serious adverse events, not related to SMV: acute sinusitis (N = 1) and phlebitis (N = 1) 3 grade 3/4 adverse events were considered related to SMV (all grade 3, all in W24 group): asthenia (N = 2) and increased serum bilirubin (N = 1) Asselah T. PLoS One 2017 Jan 5;12(1):e0168713 OSIRIS
OSIRIS Study: SMV + PEG-IFN + RBV in genotype 4 Summary Overall, 51% of patients with genotype 4 met the eligibility criteria for shortening treatment duration (W2 virologic response), and were assigned to receive 12 weeks of SMV + PEG-IFN + RBV SVR12was 97% (33/34) in this sub-group Genotype 4 subtype, sex, geographical region, and race did not appear to affect eligibility SVR12was 82% in patients assigned to receive 24 weeks of SMV + PEG-IFN + RBV The safety profile of SMV in patients with genotype 4 is comparable to previous data in patients with genotype 1 Asselah T. PLoS One 2017 Jan 5;12(1):e0168713 OSIRIS