SMV PEG-IFN RBV Study Genotype 1 Patients
Explore the ASPIRE study focusing on the use of SMV, PEG-IFN, and RBV in genotype 1 experienced HCV patients, examining dosages, outcomes, and baseline characteristics. The study aims to achieve SVR24 with significant differences in treatment groups. Research data and important details from the study are presented in a comprehensive manner.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
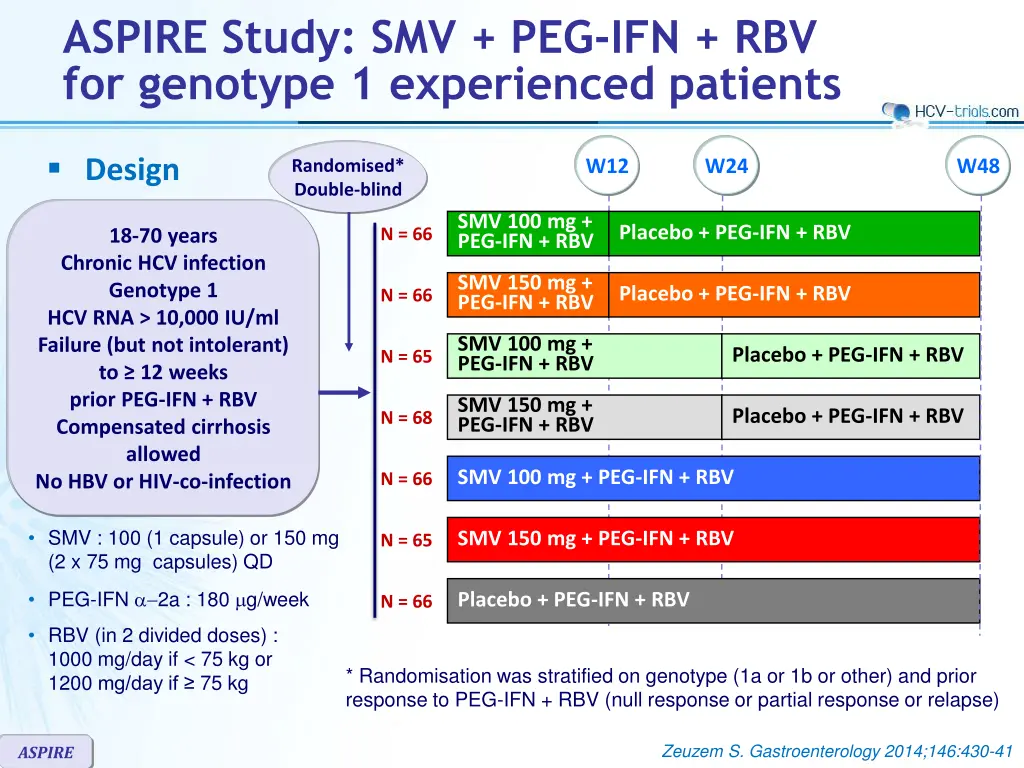
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients Design W12 W24 W48 Randomised* Double-blind SMV 100 mg + PEG-IFN + RBV Placebo + PEG-IFN + RBV N = 66 18-70 years Chronic HCV infection Genotype 1 HCV RNA > 10,000 IU/ml Failure (but not intolerant) to 12 weeks prior PEG-IFN + RBV Compensated cirrhosis allowed No HBV or HIV-co-infection SMV 150 mg + PEG-IFN + RBV Placebo + PEG-IFN + RBV N = 66 SMV 100 mg + PEG-IFN + RBV Placebo + PEG-IFN + RBV N = 65 SMV 150 mg + PEG-IFN + RBV Placebo + PEG-IFN + RBV N = 68 SMV 100 mg + PEG-IFN + RBV N = 66 SMV : 100 (1 capsule) or 150 mg (2 x 75 mg capsules) QD SMV 150 mg + PEG-IFN + RBV N = 65 PEG-IFN 2a : 180 g/week Placebo + PEG-IFN + RBV N = 66 RBV (in 2 divided doses) : 1000 mg/day if < 75 kg or 1200 mg/day if 75 kg * Randomisation was stratified on genotype (1a or 1b or other) and prior response to PEG-IFN + RBV (null response or partial response or relapse) Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients Design W12 W24 W48 Randomised* Double-blind SMV 100 mg + PEG-IFN + RBV Placebo + PEG-IFN + RBV N = 66 18-70 years Chronic HCV infection Genotype 1 HCV RNA > 10,000 IU/ml Failure (but not intolerant) to 12 weeks prior PEG-IFN + RBV Compensated cirrhosis allowed No HBV or HIV-co-infection SMV 150 mg + PEG-IFN + RBV Placebo + PEG-IFN + RBV N = 66 SMV 100 mg + PEG-IFN + RBV Placebo + PEG-IFN + RBV N = 65 SMV 150 mg + PEG-IFN + RBV Placebo + PEG-IFN + RBV N = 68 SMV 100 mg + PEG-IFN + RBV N = 66 SMV 150 mg + PEG-IFN + RBV N = 65 Placebo + PEG-IFN + RBV N = 66 Objective : SVR24(HCV RNA < 25 IU/ml) by intention to treat. Significant difference in the overall population at 5% overall significance level, power 89%. Comparison SMV100 vs placebo, SMV150 vs placebo, then each SMV group vs placebo Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients Baseline characteristics SMV 100 mg 24W N = 65 50 32% 92% SMV 150 mg 24W N = 68 52 37% 90% 12W N = 66 52 33% 89% 48W N = 66 50 32% 94% 12W N = 66 48 32% 92% 48W N = 65 50 26% 97% Placebo N = 66 51 36% 94% Median age, years Female White HCV RNA log10IU/ml, median HCV RNA > 800,000 IU/ml Genotype 1a Genotype 1b Metavir F3 / F4 (%) IL28B CC Response to prior PEG-IFN + RBV Null response Partial response Relapse 6.5 6.7 6.6 6.6 6.6 6.6 6.6 88% 39% 59% 22 / 11 16% 91% 44% 54% 25 / 21 17% 88% 39% 60% 21 / 21 17% 86% 45% 55% 17 / 20 12% 85% 45% 52% 16 / 19 18% 83% 36% 64% 11 / 20 20% 83% 41% 59% 20 / 16 22% 24% 35% 41% 25% 35% 40% 27% 33% 39% 26% 35% 39% 25% 35% 40% 26% 34% 40% 24% 35% 41% Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients Protocol-defined virologic stopping rules Patients with an insufficient response to treatment, ie, not achieving 1 log10reduction from baseline in HCV RNA at W4, 2 log10reduction from baseline in HCV RNA at W12, or HCV RNA 25 IU/ml detectable at W24 or W36, discontinued all study medication (SMV/placebo and PEG- IFN/RBV) Study medication was also discontinued if viral breakthrough (confirmed, on treatment increase in HCV RNA of > 1 log10IU/ml from the lowest level reached, or confirmed HCV RNA > 100 IU/ml in patients with HCV RNA previously < lower limit of quantification of 25 IU/ml or undetectable) at any time point during treatment Premature discontinuation N = 39 (8,4%) : withdrawal of consent, N = 21, lost to follow-up, N = 12, adverse event, N = 1, other, N = 5 Discontinuation of study medication SMV/placebo + PEG-IFN + RBV, N = 134 (29%) SMV/placebo only, N = 7 (1,5%) Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients SVR24(HCV RNA < 25 IU/ml) % 100 80 72.1 69.7 75 66.2 66.7 60.6 50 22.7 25 N 66 65 66 66 68 65 66 12W 24W 48W 12W 24W 48W Placebo SMV 100 mg SMV 150 mg SVR24: 60.6% to 80% for SMV arms versus 22.7% for placebo (p < 0.001) ; 65.5% for SMV 100mg and 72.9% for SMV 150 mg (p < 0.001 for both groups versus placebo); 68.2% for SMV 12W versus 69.2% for SMV 24W versus 70.2% for SMV 48W Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients SVR24(HCV RNA < 25 IU/ml) according to prior response to PEG-IFN + RBV Placebo SMV 100 mg SMV 150 mg % 100 85 85 75 75 57 51 46 50 37 19 25 9 N 27 78 78 23 67 68 16 49 50 Relapse Null response Partial response SVR24: 60.6% to 80% for SMV arms versus 22.7% for placebo (p < 0.001) ; 65.5% for SMV 100mg and 72.9% for SMV 150 mg (p < 0.001 for both groups versus placebo); 68.2% for SMV 12W versus 69.2% for SMV 24W versus 70.2% for SMV 48W Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients SVR24(HCV RNA < 25 IU/ml) by genotype, according to prior response to PEG-IFN + RBV Placebo SMV 100 mg SMV 150 mg % 100 89 88 85 84 82 75 68 58 56 56 50 42 40 39 33 33 33 25 13 7 0 N 12 15 34 1a 44 33 1a 45 8 15 23 1a 44 25 1a 43 7 9 24 1a 25 26 1a 24 1a 1b 1b 1b 1a 1b 1b 1b 1a 1b 1b 1b Relapse Null response Partial response SVR24for SMV 150 mg : 63.1% for genotype 1a (60.9% if Q80K+ and 66.1% if Q80K-) versus 80.4% for genotype 1b Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients SVR24in SMV 150 mg group according to other characteristics and prior response to PEG-IFN + RBV Metavir F4 Relapse : 73% Prior partial response : 82% Prior null response : 31% IL28B genotype CC : 88% CT : 74% TT : 61% Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients Treatment failure SMV 100 mg SMV 150 mg Placebo Viral breakthrough* Relapse Meeting virologic stopping rule 24 (12.2%) 21 (10.7%) 18 (9%) 17 (8.5%) 1 (1.5%) 12 (44.4%) 34 (52%) 18 (4.5%) * Generally before or at W12 NS3 emerging mutations Viral breakthrough : 41/42 Relapse : 34/35 Mostly R155K or D168V alone or a combination of Q80K or Q80R, R155K, and/or D168E mutations Genotype 1a : mainly R155K ; genotype 1b : mainly D168V Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients Adverse events, N (%) SMV 100 mg 24W N = 65 SMV 150 mg 24W N = 68 12W N = 66 48W N = 66 12W N = 66 48W N = 65 Placebo N = 66 Adverse event leading to discontinuation SMV/placebo SMV/placebo + PR 10.6% 9.1% 9.1% 6.2% 6.2% 4.6% 7.6% 6.1% 3.0% 7.6% 6.1% 4.5% 10.3% 10.3% 2.9% 10.8% 9.2% 7.2% 4.5% 4.5% 4.5% Grade 3-4 adverse event Serious adverse event 32% 4.5% 32% 7.7% 20% 4.5% 36% 10.6% 35% 7.4% 39% 12.3% 26% 6.1% Most frequent adverse event Fatigue Headache Pruritus Influenza-like illness Neutropenia 46% 27% 29% 35% 24% 43% 29% 40% 37% 23% 52% 35% 32% 32% 23% 39% 44% 30% 24% 27% 41% 38% 27% 27% 27% 43% 37% 37% 22% 31% 44% 36% 17% 20% 17% Other AE of clinical interest Hepatobiliary Rash (any type) Anemia 8% 20% 23% 3% 22% 17% 5% 27% 18% 5% 26% 15% 15% 27% 24% 9% 39% 20% 5% 18% 20% Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE
ASPIRE Study: SMV + PEG-IFN + RBV for genotype 1 experienced patients Summary Simeprevir once daily in combination with PEG-IFN + RBV achieved significantly improved SVR rates compared with placebo plus PEG-IFN + RBV in patients infected with HCV genotype 1 who failed to respond to previous PEG- IFN + RBV therapy Higher SVR24rates were seen in patients with a prior partial or null response treated with SMV150 mg compared with SMV 100 mg. This trend was also observed across most subgroups No consistent beneficial trend for treatment duration > 12W with SMV SVR24with SMV 150 mg was higher in genotype 1b than 1a among patients with a prior null or partial response SVR24in patients with cirrhosis in the SMV150 mg group were substantially higher than in the placebo group (62% versus 0%, respectively) Viral breakthrough and relapse tended to be less common with SMV 150 mg versus 100 mg. Almost all failures had emerging NS3 resistance mutations No difference in incidence or severity of adverse events with SMV vs placebo No increase in anemia. Rash and neutropenia were more common with SMV Transient mild hyperbilirubinemia with SMV (inhibition of OATP1B1) Zeuzem S. Gastroenterology 2014;146:430-41 ASPIRE