Soil Composition and Types in Agricultural Science
Learn about the composition of soil in agricultural science, including its components like mineral particles, organic matter, water, and air. Explore the ideal soil composition and classification based on clay content. Discover the characteristics of sandy and clay soils, their textures, and how they affect drainage and nutrient retention.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Leaving Certificate Agricultural Science Soil Composition Produced for IASTA by Humphrey Jones
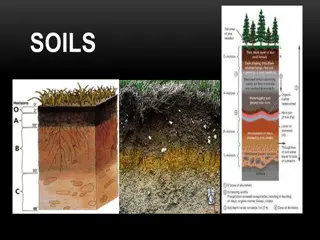
Introduction Soils are made up with the following components. Mineral particle (Sand, Silt and clay) Dead organic matter (Humus) Living organic matter (Plant roots and bacteria) Water Air Mineral salts
Composition of Soils 45% 25% 5% 25% Air Mineral Matter Water Other Material Above illustrates the ideal composition of soil, 25% Air, 25 % H2O, 45% Mineral Matter & 5% O.M.
Soil Texture The texture of a soil depends on the relative mixture of sand, silt and clay particles. The most common method of classifying soils is based on the percentage clay in the soil. E.g. Soils that contain 0 5 % clay are known as sandy soils. (More on this later) The particles in the soil are classed on their size. Anything over 2mm in diameter are referred to as pebbles or stones.
Soil Texture 2 Particles from 2 mm to 0.5 mm are called sand particles. From 0.5 mm to 0.002 mm are called silt particles. Any particle under 0.002 mm are referred to as clay. Sand and Silt are similar in composition and are formed by physical breakdown of rocks. Clay particles are formed by both physical and chemical breakdown of rocks.
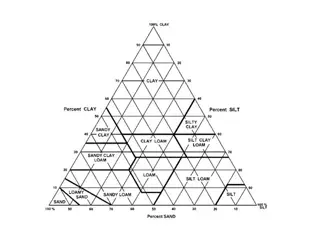
Soil Classification As mentioned before soils are classified by the amount of clay in the soil. 0 5 % Clay Sandy Soil 5 10 % Clay Sandy Loam 10 20 % Loam 20 30 % Clay Loam 30 40 % Clay Soil 40 % Up Heavy Clay Soil A more common and accurate way at looking at the type of soil is by using a soil triangle.
Soil Types Sandy Soils Have large air holes. Free Draining soils Is easy to work with (light) Dries out quickly Minerals are easily leached. Poor soil with little or no nutrients. Is a warm soil.
Soil Types Clay Soils Holds water easily. This protects from leaching of minerals. Is naturally fertile soil. Very poor drainage, which can lead to water logging. Is a cold soil.
Soil Types Loam Soils Intermediate characteristics of both clay and sandy soils. More advantages and fewer disadvantages than sandy or clay soils. A good mixture is 40 % Sand, 40 % Silt and 20 % clay. Notes: While the nature of soil depends on the particle composition, the amount of humus in the soil is also a major factor
Learning Check What materials are found in soil? How are particles classified as sand, silt and clay? How can you determine the texture of a soil? What are the characteristics of a sandy soil? What are the characteristics of a clay soil? What do you call a soil with intermediate qualities?
Activity Assessing Soil Texture Using Two Methods
Humus Humus is the product of the breakdown of organic matter. It is the remains of dead animals and plants. Fully decayed material forms CO2, Water and Mineral salts. But the incomplete product is a dark sticky material called HUMUS. Normal sandy soils as mentioned before are free draining and soil water could leach out (including the minerals dissolved in it).
Humus - 2 Humus in the soil absorbs the water and can greatly improve the quality of otherwise poor (sandy) soils. Both clay and humus can hold minerals in the soil. The minerals are in the form of Cations (Positive Ions e.g. N+ or K+ ions. The ability of a soil to maintain mineral ions in the soil is known as Cation Exchange Capacity.
Why Humus is Important to Soils Humus contains minerals (the type and amount depend on the source of the humus). It holds minerals in the soil due to its high cation exchange capacity. It improves and strengthens the crumb structure of heavy soils. It forms clay - humus complexes, which improves soil stability. Its dark colour improves the warming capabilities of the soil (i.e. it is able to absorb more heat from the sun) Can make the soil more acidic (an advantage and disadvantage depending on the use of the soil).
Categories of Humus There are two categories of humus: Fast decaying and Slow decaying. Fast Decaying Humus: Usually formed from soft parts of plants (cellulose based material) Important as food source for earthworms and bacteria. The material decays in a matter of months.
Categories of Humus Slow Decaying Humus: Usually formed from hard parts of plants (lignin based materials) It is important for soil improvement (as outlined earlier) Decays over a period of several years and forms stable complexes with clay particles.
Soils Classified by Humus Content Soils are also classified by the amount of humus in the upper horizons. < 10 % O.M. Mineral soil. 10 17 % Humus soil 17 35 % Slightly peaty soil 35 50 % Peaty soil > 50 % Peat (Turf) Note: O.M stands for Organic Matter.
Soil Micro-organisms The micro-organisms in the soil include bacteria, fungi and algae but bacteria are the most important group. The most important function of bacteria is the breakdown of organic matter into humus. Also play a major role in the Nitrogen cycle (More later) Also important in the creation and destruction of carbon in the soil.
Earthworms The earthworm plays a major role in the structure of the soil. The number of earthworms is an indication of the fertility of the soil. In good (fertile) soil there may be as many as 150-200 worms per metre squared. That is the same as 1.5 2 million worms per hectare.
Earthworms - 2 Earthworms improve the soil in the following ways: 1. They eat their way through the soil and mix the ingested material with mucus in their guts. This helps to improve soil crumb structure. 2. Depositing soil in different places and mixing horizons. 3. Improve drainage of heavy clay soils 4. Introduces more air into the soil. 5. When they die the further increase the amount of organic matter.
Learning Check What is humus? What is meant by cation exchange capacity? Why would humus be added to sandy soils? Why is humus important in soils? What are micro-organisms important in soils? Earthworms are important for soils. Give examples of why they are important.
Soil Water Water is important in the soil for some obvious reasons: 1. Required for the growth of plants and animals (like earthworms and even bacteria) 2. All chemical reactions that take place in the soil require water for them to proceed. 3. But too little or too much water can be harmful to the soil and its inhabitants.
Soil Water - 2 Flooding When all available space in the soil is taken up be water and there is excess on the surface of the soil. Water logging When all available spaces are taken up by water in the soil but there is no excess on the surface of the soil. The presence of the water is not the problem here but the resultant absence of air. The area where all the air spaces is taken up by water is called the Water Table .
Field Capacity Field capacity is the amount of soil moisture or water content held in soil after excess water has drained away. This is the ideal condition of soil . If the water level in the soil is less than the field capacity, then the amount of water required is the soil water deficit. Clay soils can hold more water and are said to have a greater field capacity. Sandy soils have poor field capacity.
Wilting Point Wilting Point Is the condition of a particular plant when it has extracted all the available water from the soil. It varies for each plant as each plant has different requirements. Different plants have different wilting points. The point at which the plant dies is known as the permanent wilting point.
Activity Measuring the amount of water in a sample of soil.
Soil Air Air is required in the soil for the growth of roots, bacteria and earthworms. It is essentially the same in composition as atmospheric air 78 % Nitrogen 21 % Oxygen 0.03 % Carbon Dioxide 0.97 % Other gases Plants use O2 for the uptake of minerals from the soil.
Soil Air - 2 Most of the useful bacteria in the soil are aerobic (i.e. they need Oxygen to live) Anaerobic bacteria do not need oxygen. Certain types of bacteria can use the Nitrogen in the soil and change it into nitrates and other compounds which are useful to the plant. These bacteria are called Nitrogen Fixing Bacteria and include Nitrobactor and Azotobacter. Sandy soils contain more air than clay soils as a general rule.
Learning Check Why is water important in the soil? Define the terms: Flooding Field capacity Wilting point Why is air important in the soil? What do you call the type of bacteria that utilise nitrogen in the soil air? What do you think would happen if a field was flooded and there was no air available? What type of soil contains a lot of air spaces?
Mineral Nutrients These are not to confused with mineral particles (sand, silt and clay) but are elements or compounds that are either bound chemically to clay particles or are dissolved in water in the soil. Plants require them as nutrients for growth and repair of their cells and also for certain chemical reactions to take place.
Mineral Nutrients - 2 They are classified by their amount of use by plants. The minerals required in large amounts are called Macro Nutrients. Examples are N, P, K, Ca and Mg. Minerals required in small amounts are called Micro Nutrients. Examples are Fe, Mn, Cu, and B. They are also known as minor elements or trace elements.
Soil Temperature Water is very slow to attract heat This means that soil with a lot of water in it harder to heat up than dry soil. In fact 1kg of water needs more than ten times the heat to raise its temperature by 1 degree, as does 1 kg of dry soil. Clay soils, as mentioned earlier can attract more heat than sandy soils, and therefore need more heat to raise its temperature.
Soil Temperature - 2 This is why clay soils are referred to as cold soils and sandy soils are called warm soils. On average, clay soils need 50 % more heat than sandy soils to raise their temperatures by 1 degree. Aspect, colour and altitude also affect the heating capabilities of a soil.
Soil pH pH is a measure of the acidity of a soil. It is important to consider because plants require certain pH s to grow well. If pH changes the plant may not be able to produce as much, or may in fact die as a result. pH also affects the ability of minerals to be absorbed by plants Earthworms and soil bacteria thrive in neutral or near neutral conditions. In soils, pH ranges mainly from 4 to 9.
Soil pH - 2 Soils can be classified by their pH values < 4.5 = Very Acidic 4.6 5.2 = Strongly acidic 5.3 5.9 = Moderately acidic 6.0 6.5 = Slightly acidic 6.6 6.9 = Near Neutral 7.0 = Neutral 7.1 7.5 = Slightly Alkaline 7.6 8.3 = Moderately alkaline.
Treatment of Acidic Soils In Ireland, soils tend to become acidic with time, and most soils need to be limed (increase the pH) every four years or so. Lime is Calcium Carbonate and is a base. The treatment of lime will increase the pH of the soil. Different plants require pH re quirements.
Chemical Properties of Clay and Humus Clay and humus are colloids. They act like large negative ions and they attract other ions (+) like Ca++, Na+, K+ NH4+ etc. This prevents the loss of these minerals by leaching. If as many ions are taken up by the colliods, the soil is said to be base saturated. Clay particles often will stick together forming crumb structures. This crumbing is called flocculation.
Flocculation Flocculated soils are better soils because: There are large air spaces Have better drainage Is warmer Is easier to cultivate. Flocculation can be increased by adding Ca++ or Mg++ ions. This causes the formation of DIVALENT Ions. This means one part is + and another -. Divalent Ions attract each other.
Base (Cation) Exchange Cations (+) attached to clay particles can be exchanged for others if there concentration is high enough. This is important because it stores ions like Ammonia (NH4+) and Potassium (K+) in the soil. However, these ions usually replace Ca++ ions which can result in a reduction in pH and increase leaching! Lime is added periodically to soils to add Ca++ and increase the soil pH.