Solid Compound Purification by Sublimation: Techniques & Applications
Learn about the process of sublimation for purifying solid compounds, its significance in chemistry, and practical applications. Sublimation involves the direct transition of a substance from solid to gas phase and is utilized by chemists for purifying various compounds like menthol, naphthalene, iodine, and ammonium chloride. Discover the procedure for sublimation, its endothermic nature, and its distinction from evaporation. Explore how sublimation is a crucial technique for obtaining highly pure substances in the field of chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
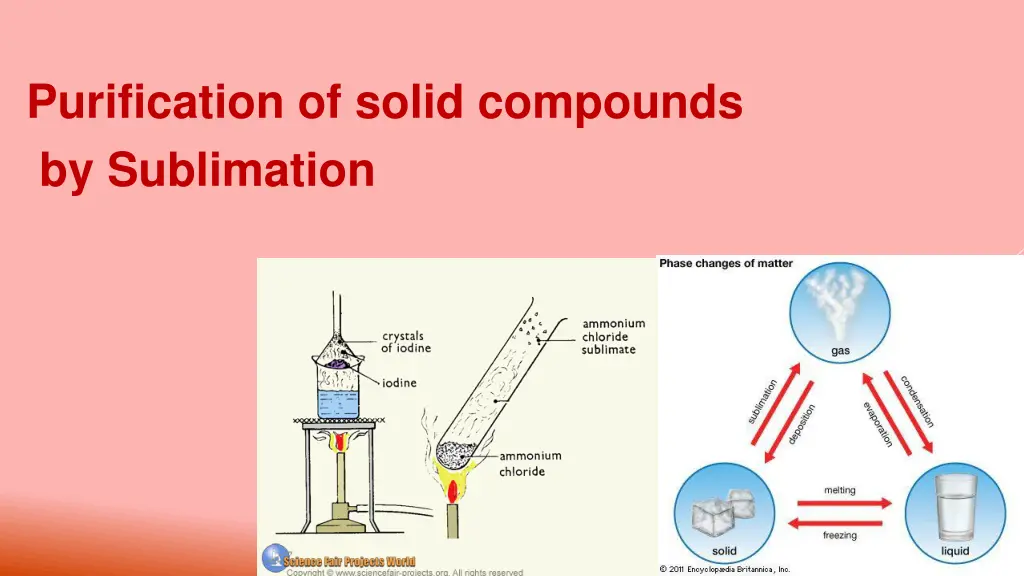
Purification of solid compounds by Sublimation
Sublimation is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase.
Sublimation is an endothermic phase transition that occurs at temperatures and pressures below a substance's triple point in its phase diagram. the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in equilibrium.
For some substances, such as carbon and arsenic, sublimation is much easier than evaporation from the melt, because the pressure of their triple point is very high, and it is difficult to obtain them as liquids.
Sublimation is a technique used by chemists to purified solid compounds, like menthol, naphthalene, iodine, ammonium chloride, etc. The reverse process of sublimation is desublimation or deposition, in which a substance passes directly from a gas to a solid phase.
Pfeil SO.svg To Solid Liquid Gas Solid-solid transformation Solid Melting Sublimation From Boiling/evapo ration Liquid Freezing Gas Deposition Condensation
Procedure: Weight 1g of impure ammonium chloride compound and put it in a crucible. Weight a pure, dry and empty cover. Cover the crucible, heat for half hour. Cool in desiccators. Weight the cover with pure sublimate compound. Calculation %purity = (wt. of pure / wt. sample) * 100 %impurity = (wt. of impure / wt. sample) * 100 Wt. pure + Wt. impure = wt. sample %purity + %impurity = 100
Calculation %purity = (wt. of pure / wt. sample) * 100 %impurity = (wt. of impure / wt. sample) * 100 Wt. pure + Wt. impure = wt. sample %purity + %impurity = 100






















