Solutions and Concentration Units
Explore the world of solutions, the role of solvents and solutes, why solutions form, different concentration units like molarity and molality, calculations involved, colligative properties such as vapor pressure lowering, and how Raoult's Law explains changes in boiling and freezing points in solutions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
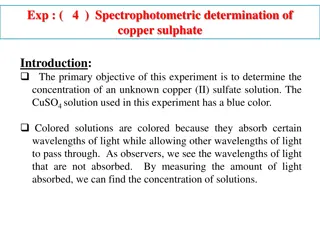
Solutions Homogeneous Mixture of 2 or more Substances
Some Terminology Solvent The substance that does the dissolving Solute The substance that gets dissolved Solution The resulting mixture
Why Isnt Everything Soluble in Everything?
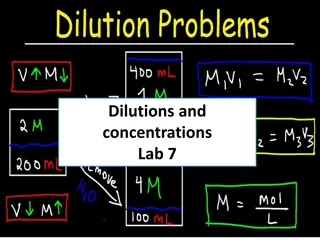
Some Concentration Units Molarity - # mol solute / # L solution Molality - # mol solute / # kg solvent Mole Fraction of substance - # mol / total mol Mass Percent of Solute 100% (solute mass / solution mass)
Time to do some calculations! Calculation of concentration units Conversion of one concentration unit to another
Colligative Properties Vapor Pressure Lowering Boiling Point Elevation Freezing Point Depression Osmotic Pressure
Why Does Vapor Pressure Lowering Change the Boiling and Freezing Points?
Raoults Law Applied to Two Volatile Components in a Solution