Steam and Its Properties
Explore the commonly recognized forms of steam, including wet steam, saturated steam, and superheated steam. Learn about saturation, boiling point, quality, and dryness fraction in steam engineering. Discover the different phases of steam and their characteristics in detail.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
MEDICAL ENGINEERING DEPT SECOND YEAR LECTURE BY: PROF. M. REFAAT DIAB 1
STEAM Commonly Recognized Forms of Steam 2
STEAM Commonly Recognized Forms of Steam 3
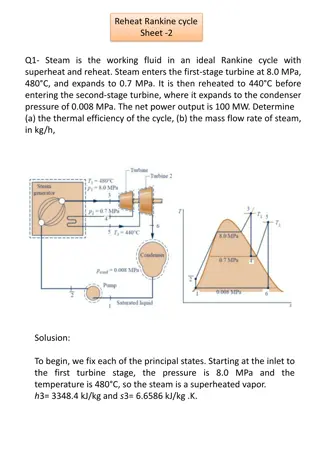
DEFINITIONS Saturation Saturationdefines a condition in which a mixture of vapor and liquid can exist together at a given temperature and pressure. Saturation Temperature Or Boiling Point. Saturation Temperatureisthe temperature at which vaporization (boiling) starts to occur for a given pressure. 4
STEAM Commonly Recognized Forms of Steam Wet steam Wet steamcontains both condensed hot-water particles and steam and is considered a two-phase mixture. Wet steam is difficult to measure with any of the technologies currently available, and accuracies of the water content can vary with the testing method. Saturated steam Saturated steamoccurs at a particular pressure and corresponding temperature when a state of equilibrium exists between liquid and vapor states.
Commonly Recognized Forms of Steam (Continue..) Superheated steam Superheated steam is at a higher temperature than the saturation temperature at a constant pressure. In a boiler, steam is generally saturated. As steam leaves a conventional boiler, a pressure drop is created by the flow. This drop in pressure, along with a relatively constant temperature of the steam leaving the boiler, results in superheated steam. This superheated steam conforms to ideal gas laws. 6
Commonly Recognized Forms of Steam (Continue..) Quality, Dryness Fraction, x Quality (x) is the ratio of the mass of the vapor to the total mass of both vapor and liquid . If the mass of vapor is 0.2 kg and the mass of the liquid is 0.8 kg, the quality is 0.2 or 20%. Quality is an intensive property. 7
Quality, Dryness Fraction, x (Continue.) Quality has meaning when the substance is in a saturated state only, at saturation pressure and temperature. x = mvapor /(mvapor + mliquid) 8
Commonly Recognized Forms of Steam (Continue..) Positive Pressure Steam Steam is typically generated and distributed at a positive pressure. In most cases, this means that it is supplied to equipment at pressures above 0 MPag and temperatures higher than 100 C. Heating applications for positive pressure steam can be found in food processing factories, refineries, and chemical plants to name a few. Saturated steam is used as the heating source for process fluid heat exchangers, reboilers, reactors, combustion air preheaters, and other types of heat transfer equipment. 10
Critical Point Critical Pointisa point at which the saturated-liquid and saturated-vapor states are identical. The temperature, pressure, and specific volume at the critical point are called the critical temperature, critical pressure, and critical volume. A constant pressure process greater than the critical pressure There is no definite change in phase from liquid to vapor and no definite point at which there is a change from the liquid phase to the vapor phase. For pressures greater than the critical pressure, the substance is usually called a liquid when the temperature is less than the critical temperature, and a vapor when the temperature is greater than the critical temperature. . 11
Definitions (Continue) Fusion Fusionis the process of melting. The heat added to melt ice into a liquid is called the latent heat of fusion. Sublimation Sublimation is a process in which the transition between the solid phase and the vapor phase occurs directly, without passing through the liquid phase. Further heat transfer would result in superheating the vapor. Triple point Triple point, defined as the state in which all three phases may be present in equilibrium. 13
CONDENSATION If heat is removed at a constant pressure from a saturated vapor, condensation will occur and the vapor will change phase to liquid. The processes of vaporization and condensation are the exact opposite of each other. Similarly, freezing is the opposite process of melting and fusion. Sublimation also has an opposite process in which a gas goes directly to solid, but this process is not normally referred to with a unique term. 14
Vacuum Steam The use of steam for heating at temperatures below 100 C, traditionally the temperature range in which hot water is used, has grown rapidly in recent years. When vacuum saturated steam is used in the same manner as positive pressure saturated steam, the temperature of the steam can be quickly changed by adjusting the pressure, making it possible to achieve precise applications using hot water. A vacuum pump must be used in conjunction with the equipment, because merely reducing the pressure will not drop it to below atmospheric pressure. temperature control unlike 15
STEAM APPLICAIONS 1. STEAM FOR PROCESS HEATING 2. STEAM FOR ATOMIZATION 3. HEAT TREATMENT WITH STEAM FOR STERILIZATION 4. STEAM FOR POWER GENERATION 5. STEAM FOR HUMIDIFICATION 6. STEAM FOR DRYING 7. THERMAL ENERGY STORAGE, TES 16
STEAM APPLICAIONS (C0ntinue) 8. STEAM FOR CLEANING 9. STEAM FOR MOISTURIZATION 10. USE OF STEAM AND SUPERHEATED STEAM IN HEAT TREATING AND OTHER MATERIALS APPLICATIONS 11. HIGH-TEMPERATURE APPLICATIONS OF STEAM 12. STEAM AND SUPERHEATED STEAM FOR CHEMICAL CRACKING 13. GASIFICATION BY STEAM 14. HIGH-TEMPERATURE MATERIALS TESTING 17
STEAM FOR PROCESS HEATING (Continue.) Steam can be used either for direct heating or indirect heating. a. Direct Heating In direct heating, steam is directly injected in the substance which is to be heated. Care should be taken that proper mixing takes place to ensure uniform heating. It is also essential to take care that no temperature overshoots are observed. In pharmaceutical or food and beverages industry, steam of highest purity (safe to be consumed by humans) should always be used for direct heating purposes. b. Indirect Heating Steam is used to heat the product with the help of heat exchangers so that the product does not come physically in contact with steam. 18
2. STEAM FOR ATOMIZATION (Continue.) Atomization means breaking in to tiny particles. In burners, steam is used for the purpose of atomizing the fuel. This ensures a larger surface area of the fuel available for the combustion. As a result of atomization, soot formation is minimized and overall efficiency of combustion goes up. 19
3. HEAT TREATMENT WITH STEAM FOR STERILIZATION High-temperature steam is used for sterilization in autoclaves. An autoclave is a device that uses pressurized steam to sanitize and sterilize materials and equipment. Since the steam is under pressure, it can reach these and higher temperatures. Autoclaves are commonly found in microbiology laboratories, hospitals, dental offices and similar environments for sterilization and other uses. 20
4. STEAM FOR POWER GENERATION Power-generation plants produce high temperature steam from the combustion or reaction of a fuel to turn a turbine generator (creating rotational energy from thermal energy). These steam temperatures are quite high and can be as much as 600 800 C in the case of supercritical boilers. Since the primary difference in these systems is the fuel for creating the high- temperature steam, the common types of power plants utilize steam similarly. Steam is being used for the purpose of power generation in form of electricity. The steam power plants work on the Rankine Cycle. In Rankine cycle, superheated steam is generated and then taken to steam turbine. 21
5. STEAM FOR HUMIDIFICATION Steam Humidifier in Air Duct Steam is used to humidify air within an air duct before the air is distributed to other regions of a building. 22
STEAM FOR HUMIDIFICATION (Continue..) Many large commercial and industrial facilities, especially in colder climates, use low pressure saturated steam as the predominant heat source for indoor seasonal heating. HVAC coils, often combined with steam humidifiers, are the equipment used for conditioning the air for indoor comfort, preservation of books and records, and infection control. When the cold air is heated by the steam coils, the relative humidity of the air drops, and it must then be adjusted to normal levels with addition of a controlled injection of dry saturated steam into the downstream air flow. 23
STEAM FOR HUMIDIFICATION (Continue..) Using steam for the purpose of humidification offers added advantages over other media. There are different types of humidifiers from evaporating humidifiers to ultrasonic ones to suit different applications. Maintaining humidity is a crucial aspect of HVAC systems as humidity lower or higher than desired has adverse effects on humans, machines and materials. Humidity lower than desired might lead to drying of mucus membranes which ultimately results in respiratory distress. Low humidity also leads to increased static electricity problems which might damage the costly equipment. 24
6. STEAM FOR DRYING Steam is used to remove moisture from the product. Conventionally, hot air is used for product drying. Using steam to dry makes the system simple, easy to control drying rates and compact. The overall capital investment is also low. Use of steam is cheaper on operational basis compared to hot air. It is also a safer alternative. The use of steam for drying purpose ensures a better product quality when compared with hot air. 25
7. THERMAL ENERGY STORAGE, TES In industrial applications, steam is used for energy storage. This energy is introduced and extracted by heat transfer, usually through pipes. Steam is an excellent reservoir for thermal energy because of the high latent heat of vaporization of water (2260 kJ/kg at 100 C). Steam gas can hold more than five times more energy per kg at the same temperature as air. This provides an excellent medium for energy transfer. 26
TES (Continue..) The effective integration of TES into heating and cooling energy systems can lead to benefits such as: Reduction of energy consumption Increase of energy efficiency Increased energy security Increased energy reliability Reduction of energy costs Reduction of green house gases, GHG, emissions Thermal energy storage should be used properly; for example for balancing thermal supply and demand, integrating renewable energy, or recovering waste heat. Thermal energy storage, is classified into: Centralized TES. Decentralized TES. 27
TES (Continue..) Centralized energy storages can be found in district heating and cooling networks, large industrial plants, combined heat and power plants and renewable energy power plants. Decentralized energy storages are found commonly in domestic and commercial buildings, where it s used to store solar energy for hot water and space heating applications. The application of thermal energy storage with renewable energy sources, waste heat, or surplus energy production can replace heat or cold generation from fossil-fuels, reducing greenhouse gas (GHG) emissions and reducing the need for thermal power capacity of the generators. Energy storage should be used properly; for example for balancing thermal supply and demand, integrating renewable energy, or recovering waste heat. 28
TES (Continue..) Methods of Thermal Energy Storage Thermal energy storage applications may use different material properties to achieve energy storage. TES can be classified in three types: Sensible (e.g., water and rock) Storage happens when the temperature of a material is raised or lowered. Latent (e.g., water/ice and salt hydrates) Latent storage occurs when the phase of a material is changed (solid to liquid or liquid to vapor) without a change in temperature. Thermo-chemical reactions (e.g., chemical reactions and sorption processes). A chemical reaction or a sorption process, takes place on the surface of a material. In all cases heat can be either absorbed or released from the material. 29
8. STEAM FOR CLEANING Steam is used to clean a wide range of surfaces. One such example from industry is the use of steam in soot blowers. Boilers that use oil or coal as the fuel source must be equipped with soot blowers for cyclic cleaning of the furnace walls and removing combusted deposits from convection surfaces to maintain boiler capacity, efficiency, and reliability 30
9. STEAM FOR MOISTURIZING Steam is sometimes used to add moisture to a process while at the same time supplying heat. Steam is used for moisturization in the production of paper, so that paper moving over rolls at high speed does not suffer microscopic breaks or tears. Often mills that produce animal feed in pellet form use direct-injected steam to both heat and provide additional water content to the feed material in the conditioner section of the mill. The moisturizing of the feed softens the feed and partially gelatinizes the starch content of the ingredients, resulting in firmer pellets. 31
10. USE OF STEAM AND SUPERHEATED STEAM IN HEAT TREATING AND OTHER MATERIALS APPLICATIONS Steam is a colorless, odorless gas with the ability to carry and transfer large amounts of thermal energy due to its high enthalpy. This high enthalpy makes steam an excellent candidate for a wide variety of applications sterilization, pharmaceuticals, energy production, drying and many more. in heat treatment, 32
APPLICATIONS OF STEAM (Continue..) 11. HIGH-TEMPERATURE APPLICATIONS OF STEAM Steam is used in a variety of areas in its various forms. Specifically, steam is used in the following areas of heat treatment and other high- temperature processes. 12. STEAM AND SUPERHEATED STEAM FOR CHEMICAL CRACKING Steam cracking is used in industrial petrochemical processes for breaking down saturated hydrocarbons into smaller hydrocarbons (mostly unsaturated). This method is used in the production of olefins (and other light alkenes), propene (or propylene) and ethene (or ethylene). 33