Stoichiometry and Molar Mass Review
Understand stoichiometry, molar mass calculations, percentage composition, empirical formula determination, and molecular formula determination from experimental data. Learn about mass, moles, and particles relationships in chemical reactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
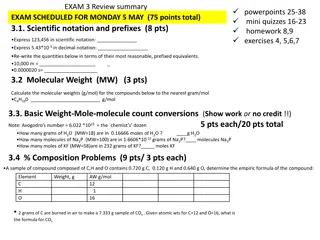
Spring Final Exam Stoichiometry Review
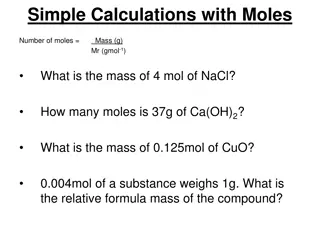
Molar Mass the mass of one mole of a substance 1 (207.2 g) = 207.2 g Pb: PbO2 239.3 g O: 2 (16.0 g) = 32.0 g 1 (1.0 g) H: = 1.0 g HNO3 63.0 g N: 1 (14.0 g) = 14.0 g O: 3 (16.0 g) = 48.0 g
percentage composition: the mass % of each element in a compound g element x 100 % of element = molar mass of compound (see calcs above) Find % composition. : : 207.2 g Pb 32.0 g O = 86.6% Pb 239.2 g 239.2 g = 13.4% O PbO2 : : : : 42.0 g N 12.0 g H 149.0 g = 28.2% N 149.0 g = 8.1% H (NH4)3PO4 31.2 g P 64.0 g O 149.0 g = 20.8% P 149.0 g = 43.0% O
Finding an Empirical Formula from Experimental Data 1. Find # of g of each element. 2. Convert each g to mol. 3. Divide each # of mol by the smallest # of mol. 4. Use ratio to find formula. A compound is 45.5% yttrium and 54.5% chlorine. Find its empirical formula. mol 1 Y 0.512 1 0.512 = 45.5 g Y mol Y 88.9 g Y mol 1 Cl 0.512 3 1.535 = 54.5 g Cl mol Cl 35.5 g Cl YCl3
To find molecular formula A. Find empirical formula. B. Find molar mass of empirical formula. C. Find x = MM molecular MM empirical D. Multiply all parts of empirical formula by x. (How many empiricals fit into the molecular?)
A carbon/hydrogen compound is 7.7% H and has a molar mass of 78 g. Find its molecular formula. mol 1 H 7.69 1 = 7.7 g H 7.7 mol H 1.0 g H mol 1 C 7.69 1 7.69 = 92.3 g C mol C 12.0 g C emp. form. CH 78 g 13 g mmemp =13 g C6H6 = 6
A compound has 26.33 g nitrogen, 60.20 g oxygen, and molar mass 92 g. Find molecular formula. mol 1 N 1.881 1 1.881 = 26.33 g N mol N 14.0 g N mol 1 O 1.881 2 3.763 = 60.20 g O mol O 16.0 g O NO2 92 g 46 g mmemp =46 g N2O4 = 2
1 mol = molar mass (in g) Mass (g) 1 mol = 22.4 L 1 mol = 22.4 dm3 MOLE (mol) Volume (L or dm3) Particle (at. or m c) Island Diagram: 1 mol = 6.02 x 1023 particles a. Diagram has four islands. b. Mass Island for elements or compounds c. Particle Island for atoms or molecules d. Volume Island : for gases only 1 mol @ STP = 22.4 L = 22.4 dm3
What mass is 1.29 mol ? Iron (II) nitrate Fe2+NO31 ( 1 mol Fe(NO3)2 ) 179.8 g 1.29 mol = 232 g How many molecules is 415 L at STP? sulfur dioxide sulfur dioxide SO2 ) 1 mol 22.4 L( ) 415 L( 1 mol 6.02 x 1023m c = 1.12 x 1025m c
aluminum sulfate aluminum sulfate What mass is 6.29 x 1024m cules ? Al3+ Al2(SO4)3 342.3 g ) SO42 ( ( ) 1 mol 6.02 x 1023m c 6.29 x 1024m c 1 mol = 3580 g At STP, how many g is 87.3 L of nitrogen gas? N2 ( ) ( ) 1 mol 28.0 g 87.3 L = 109 g 22.4 L 1 mol
During a chem. rxn.; atoms are rearranged (NOT created or destroyed!) Chemical equations must be balanced to show the relative amounts of all substances. Balanced means: each side of the equations has the same # of atoms of each element. Unbalanced CH4 + O2 > H2O + CO2 CH4 + 2O2 > 2H2O + CO2 Balanced
Cellulose reacts with oxygen gas to form carbon dioxide gas & liquid water. C6H10O5 + 6O2 6CO2 +5H2O 6 C H O 6 10 17 10 17 Balanced!!!
Nitroglycerin decomposes to form nitrogen gas, oxygen gas, carbon dioxide gas & water vapor 2 C3H5(NO3)3 3N2 + O2 + 6CO2 +5H2O 6 C H N O 6 10 6 19 10 6 18 Not Balanced!
Balancing Chemical Equat. When balancing equations, you may change coefficients as much as you need to, but you may never change subscripts because you can t change what substances are involved.
2 2 H2 (g) + O2 (g) H2O (l) 1. Atom Inventory: Reactants 2 Add coefficients to balance 3. Double check to make sure it is all BALANCED! H O Products 4 2 2 2 1 4 2
Balancing Chemical Equat. Balancing Chemical Equations practice