Structural Principles of Atomic and Ionic Sizes: Radii Scales and Trends
Explore the structural principles of atomic and ionic sizes detailing radii scales, trends, and periodic variations. Understand how atomic and ionic sizes vary based on factors such as group, period, and oxidation state, with insights into covalent and metallic radii.
Uploaded on | 4 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
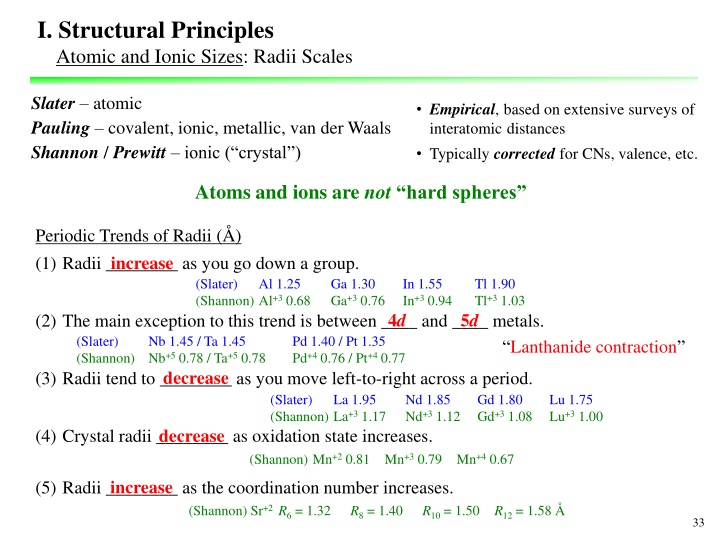
I. Structural Principles Atomic and Ionic Sizes: Radii Scales Slater atomic Pauling covalent, ionic, metallic, van der Waals Shannon / Prewitt ionic ( crystal ) Empirical, based on extensive surveys of interatomic distances Typically corrected for CNs, valence, etc. Atoms and ions are not hard spheres Periodic Trends of Radii ( ) (1) Radii ________ as you go down a group. increase (Slater) (Shannon) Al+3 0.68 Al 1.25 Ga 1.30 Ga+3 0.76 In 1.55 In+3 0.94 Tl 1.90 Tl+3 1.03 4d 5d (2) The main exception to this trend is between ____ and ____ metals. (Slater) Nb 1.45 / Ta 1.45 (Shannon) Nb+5 0.78 / Ta+5 0.78 Pd 1.40 / Pt 1.35 Pd+4 0.76 / Pt+4 0.77 Lanthanide contraction (3) Radii tend to ________ as you move left-to-right across a period. decrease (Slater) (Shannon) La+3 1.17 La 1.95 Nd 1.85 Nd+3 1.12 Gd 1.80 Gd+3 1.08 Lu 1.75 Lu+3 1.00 (4) Crystal radii ________ as oxidation state increases. decrease (Shannon) Mn+2 0.81 Mn+3 0.79 Mn+4 0.67 (5) Radii ________ as the coordination number increases. increase (Shannon) Sr+2 R6 = 1.32 R8 = 1.40 R10 = 1.50 R12 = 1.58 33
I. Structural Principles Atomic and Ionic Sizes: Metallic Radii Wells, pp. 1286-1295 Usually defined for CN = 12: ?CN Ideal CP structures (CCP, HCP with c/a = 1.63): ?12= ?M M2 Distorted CP structures (Mn, Zn, Cd, Hg, In): ?12= ?M M 2 Structures with CN < 12 (BCC, semimetals): R12 = 1.029 R8 R12 = 1.042 R6 R12 = 1.136 R4 ?12= ?CN Estimation Strategies: o Constant ?atom (Goldschmidt estimation) BCC FCC BCC vs FCC : ?atom=1 3=1 2 4 3 2?BCC 4?FCC 2 3 BCC: 2?8= FCC: 2?12= 2?BCC 2?FCC Example: Nb (BCC) aBCC = 3.300 R8 = 1.429 R12 = 1.470 (Body-Diagonal) (Face-Diagonal) R12 = 1.029 R8 (Main Group Metals, Semimetals, ...) For Mn, Hg, Ga, In, Si, Ge, Sn, Sb, Bi, Se, Te o Use alloys with close packed structures, e.g., Ag3Sb (HCP) provides R12(Sb) o Linear extrapolation of solid solutions of the element (solute) in a close packed metal 34
I. Structural Principles Atomic and Ionic Sizes: Metallic Radii Wells, pp. 1286-1295 Periodic Trends 2.80 2.40 R12 ( ) 2.00 Minimum Radii Lanthanide Contraction 6th Period (5d) 1.60 5th Period (4d) 4th Period (3d) 1.20 Increasing # Valence Electrons Increasing Zeff 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Group Number 5d Filling M M Bonding d States Filling M M Antibonding d States 800 Ucoh (kJ/mol) 4d 600 M(s) M(g) 3d 400 Maximum Cohesive Energy 200 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Group Number 35
I. Structural Principles Atomic and Ionic Sizes: Covalent Radii Wells, pp. 287-291 Defined for covalent bond orders n: RA(n) Periodic Trends RC(1): 76 pm (sp3, single bond) RC(2): 73 pm (sp2, double bond) RC(3): 69 pm (sp, triple bond) Main Group s, p Elements 250 250 Alkali Metals Li-Cs Alkaline Earth Metals Be-Ba 200 200 Single-Bond Radii: RA(1) (pm) Icosogens B-Tl Tetragens C-Pb Pnicogens N-Bi Chalcogens O-Po Halogens F-At 150 150 No 1d or 2d 100 100 No 1p Enhanced multiple bonding 50 50 Shielding of valence e s by core e s including Pauli Exclusion Principle 2 2 3 3 4 4 5 5 6 6 Period 36
I. Structural Principles Atomic and Ionic Sizes: Covalent Radii Burdett, pp. 200-207 Defined for covalent bond orders n: RA(n) Pauling: dAA(n) dAA(1) = 2RA(n) 2RA(1) Pauling: dAA(n) dAA(1) = 0.6 log n measured n = 10???1 ???? /0.6 Bond-Valence Method (I.D. Brown: Structure and Bonding in Crystals, Vol. II) Valence measure of the bonding power of an atom/ion -- number of electrons taking part in chemical bonding (covalent) -- charge of ions (ionic) ?; dAX(1) and B are empirical parameters (B 0.37 ) ???? ?AX??? Bond Valence: ???= ? measured (Bond A X) (1) Valence Sum Rule: Sum of bond valences (sAX) at each atom (A) = valence at atom A (2) Rule of Stoichiometry: Total valence of Lewis acids (cations) = Total valence of Lewis bases (anions) Brown, Altermatt, Acta Cryst. 1985, B41, 244-247 O Keeffe, Acta Cryst. 1990, A46, 138-142 Brese, O Keeffe, Acta Cryst. 1991, B47, 192-197 37
I. Structural Principles Atomic and Ionic Sizes: Covalent Radii Burdett, pp. 200-207 Bond-Valence Method (I.D. Brown: Structure and Bonding in Crystals, Vol. II) Applications: (1) Examine experimental structures for accuracy, determine oxidation states, identify bonding instabilities. (2) Locate light atoms difficult to find by X-ray diffraction. (3) Predict bond distances as an alternative to using radii. (Fe2+)(Ti4+)(O2 )3 or (Fe3+)(Ti3+)(O2 )3 FeTiO3 (Ilmenite): Al2O3-type (Corundum) d6 d0 d5 d1 dFe-O = 3 2.07, 3 2.20 dTi-O = 3 1.88, 3 2.09 ?AX1 ?????? ? ?AX= ? (B = 0.37 ) dAX(1) 1.734 1.759 1.791 1.815 Fe Fe(II)-O Fe(III)-O Ti(III)-O Ti(IV)-O Fe(II): s = 3(0.403) + 3(0.284) = 2.06 Ti(IV): s = 3(0.839) + 3(0.476) = 3.95 O: s = 0.403+0.284+0.839+0.476 = 2.00 Fe(III): s = 3(0.431) + 3(0.304) = 2.21 Ti(III): s = 3(0.786) + 3(0.446) = 3.70 O: s = 0.431+0.304+0.786+0.446 = 1.97 Ti HCP O 38
I. Structural Principles Atomic and Ionic Sizes: Covalent Radii Burdett, pp. 200-207 Bond-Valence Method (I.D. Brown: Structure and Bonding in Crystals, Vol. II) Applications: (1) Examine experimental structures for accuracy, determine oxidation states, identify bonding instabilities. (2) Locate light atoms difficult to find by X-ray diffraction. (3) Predict bond distances as an alternative to using radii. AlO(OH): Diaspore ?AX1 ?????? ? ?AX= ? O2 dAl-O1 = 2 1.85, 1 1.86 dAl-O2 = 2 1.97, 1 1.98 (B = 0.37 ) dAX(1) 1.651 Al(III)-O Al: s = 2.99 O1: s = 1.74 O2: s = 1.26 OH group O1 Where do the H atoms attach, at O1 or O2? 39
I. Structural Principles Atomic and Ionic Sizes: Ionic Radii Wells, pp. 312-321 Rion(O2 ) = 1.40 (140 pm) on most scales and is a good rule of thumb Ion Pauling ( ) Shannon ( ) Atomic ( ) ( 0.85) ( 0.69) Li+ Li+ 0.60 0.76 1.45 ( 0.85) ( 0.78) Wurtzite? (CN = 4) NiAs? (CN = 6) Na+ Na+ 0.95 1.02 1.80 ( 0.87) ( 0.82) K+ K+ 1.33 1.38 2.20 ( 0.87) ( 0.83) Rb+ Rb+ 1.48 1.52 2.35 ( 0.74) ( 0.60) Be2+ Be2+ 0.31 0.45 1.05 ( 0.85) ( 0.78) Mg2+ Mg2+ 0.65 0.72 1.50 MgO (a = 4.20 ) R(Mg2+) / R(O2 ) = 0.46 MgTe (HP?) R(Mg2+) / R(Te2 ) = 0.29 ( 0.87) ( 0.82) Sr2+ Sr2+ 1.13 1.18 2.00 ( 0.80) ( 0.80) Ba2+ Ba2+ 1.35 1.35 2.15 F F 1.36 (+0.86) 1.33 (+0.83) 0.50 Cl Cl 1.81 (+0.81) 1.81 (+0.81) 1.00 O2 O2 1.40 (+0.80) 1.40 (+0.80) 0.60 Te2 Te2 2.21 (+0.81) 2.21 (+0.81) 1.40 Anions not always close packed, but eutactic . ( Eutaxy - = well ordered ) Maximum volume (minimum anion- anion repulsion) for fixed cation- anion distance BaO (a = 5.53 ) R(Ba2+) / R(O2 ) = 0.96 BaTe (a = 6.89 ) R(Ba2+) / R(Te2 ) = 0.61 40 Phys. Rev. Lett., 1995, 74, 5232-5235.