Study of Glecaprevir/Pibrentasvir in Genotype 1, 2, 4, 5, or 6 with Compensated Cirrhosis
This study, named EXPEDITION-1, focuses on the efficacy of glecaprevir/pibrentasvir in treating HCV genotypes 1, 2, 4, 5, and 6 in patients with compensated cirrhosis. The primary endpoint is achieving SVR12, with promising results seen across different genotypes. The research provides insights into baseline characteristics, treatment outcomes, adverse events, and laboratory abnormalities. Overall, glecaprevir/pibrentasvir shows high efficacy and tolerability in this patient population.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
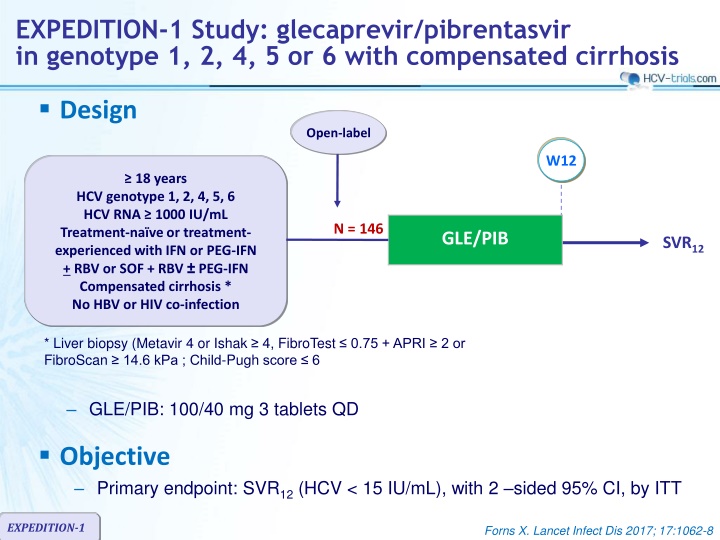
EXPEDITION-1 Study: glecaprevir/pibrentasvir in genotype 1, 2, 4, 5 or 6 with compensated cirrhosis Design Open-label W12 18 years HCV genotype 1, 2, 4, 5, 6 HCV RNA 1000 IU/mL Treatment-na ve or treatment- experienced with IFN or PEG-IFN + RBV or SOF + RBV PEG-IFN Compensated cirrhosis * No HBV or HIV co-infection N = 146 GLE/PIB SVR12 * Liver biopsy (Metavir 4 or Ishak 4, FibroTest 0.75 + APRI 2 or FibroScan 14.6 kPa ; Child-Pugh score 6 GLE/PIB: 100/40 mg 3 tablets QD Objective Primary endpoint: SVR12(HCV < 15 IU/mL), with 2 sided 95% CI, by ITT EXPEDITION-1 Forns X. Lancet Infect Dis 2017; 17:1062-8
EXPEDITION-1 Study: glecaprevir/pibrentasvir in genotype 1, 2, 4, 5 or 6 with compensated cirrhosis Baseline characteristics GLE/PIB 12W N = 146 Median age, years 60 Female, % 38 Race: White / Black, % 82 / 10 Mean BMI, kg/m2 29.2 Genotype: 1a / 1b / 2 / 4 / 5 / 6, % 33 / 27 / 23 / 11 / 1 / 5 Mean HCV RNA, log10IU/mL IL-28B CC, % 6.1 27 Child-Pugh score at screening : 5 / 6, % 91 / 9 Treatment na ve, % 75 Treatment-experienced, % IFN-based SOF-based 25 69 31 Baseline RASs : None / NS3 only / NS5A only / NS3 + NS5A, % 57 / 2 / 40 / 2 EXPEDITION-1 Forns X. Lancet Infect Dis 2017; 17:1062-8
EXPEDITION-1 Study: glecaprevir/pibrentasvir in genotype 1, 2, 4, 5 or 6 with compensated cirrhosis SVR12 (HCV RNA < 15 IU/mL),by ITT, % (95% CI) 99 100 100 100 100 (98-100) 99 100 80 60 40 20 90 31 16 2 7 146 0 GT1 GT2 GT4 GT5 GT6 Total One patient with genotype 1a and history of non-reponse to Peg-IFN + RBV relapsed at post- treatment week 8 - NS5A RASs: Y93N at baseline, Y93N, Q30R and H58D present at failure - NS3 RASs: none EXPEDITION-1 Forns X. Lancet Infect Dis 2017; 17:1062-8
EXPEDITION-1 Study: glecaprevir/pibrentasvir in genotype 1, 2, 4, 5 or 6 with compensated cirrhosis Adverse events and laboratory abnormalities, % GLE/PIB 12W N = 146 69 8 0 0 Any adverse event Serious adverse event Drug-related Adverse event leading to discontinuation Adverse events in > 10% of patients, % Fatigue Headache Pruritus Laboratory abnormalities Hemoglobin < 8 g/dL Platelet count 25-50 x 109/L AST grade 3 (> 5 x ULN) ALT grade 3 (> 5 x ULN) Total bilirubin grade 3 (> 3 x ULN) 19 14 10 0.7 1 0 0 0 Occurrence of hepatocellular carcinoma in 2 patients One patient with a history of hemophilia died 61 days after completing treatment (cerebral hemorrhage, not related to study drugs) EXPEDITION-1 Forns X. Lancet Infect Dis 2017; 17:1062-8
EXPEDITION-1 Study: glecaprevir/pibrentasvir in genotype 1, 2, 4, 5 or 6 with compensated cirrhosis Summary Glecaprevir/pibrentasvir for 12 weeks achieved a 99% SVR12 rate in patients with compensated cirrhosis and genotype 1, 2, 4, 5 or 6 Treatment was well-tolerated no elevations in alanine aminotransferase and no treatment discontinuations due to adverse events EXPEDITION-1 Forns X. Lancet Infect Dis 2017; 17:1062-8