Study on Glecaprevir/Pibrentasvir in Patients with Renal Impairment
This study examines the efficacy and safety of glecaprevir/pibrentasvir in HCV patients with renal impairment. Results show high SVR12 rates across all major HCV genotypes, well-tolerated treatment, and no virologic failures, making it a promising option for this challenging population.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
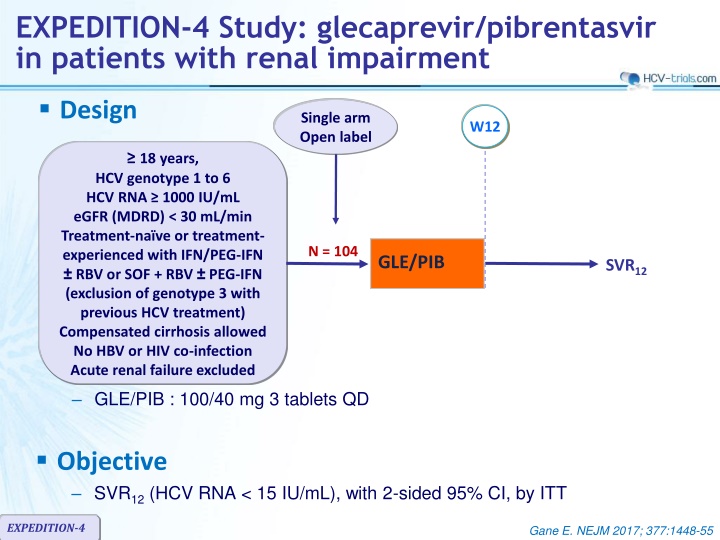
EXPEDITION-4 Study: glecaprevir/pibrentasvir in patients with renal impairment Design Single arm Open label W12 18 years, HCV genotype 1 to 6 HCV RNA 1000 IU/mL eGFR (MDRD) < 30 mL/min Treatment-na ve or treatment- experienced with IFN/PEG-IFN RBV or SOF + RBV PEG-IFN (exclusion of genotype 3 with previous HCV treatment) Compensated cirrhosis allowed No HBV or HIV co-infection Acute renal failure excluded N = 104 GLE/PIB SVR12 GLE/PIB : 100/40 mg 3 tablets QD Objective SVR12(HCV RNA < 15 IU/mL), with 2-sided 95% CI, by ITT EXPEDITION-4 Gane E. NEJM 2017; 377:1448-55
EXPEDITION-4 Study: glecaprevir/pibrentasvir in patients with renal impairment Baseline characteristics and SVR12 GLE/PIB , N = 104 Mean age, years Female, % Race : White / Black / Asian, % Median BMI, kg/m2 Genotype 1a / 1b / 1 other / 2 / 3 / 4 / 5 / 6, % Median HCV RNA, log10IU/mL Compensated cirrhosis, % Treatment-experienced, % (IFN-based / SOF-based) Chronic kidney disease stage, % eGFR 15-29 ml/min/1.73 m2 eGFR < 15 ml/min/1.73 m2(hemodialysis) Baseline polymorphism, % NS3 only NS5A only NS3 + NS5A 57 24 62 / 24 / 9 26 22 / 28 / 2 / 16 / 11 / 19 / 1 / 1 5.9 19 42 (95 / 5) 13 87 (82) 1 25 0 102/104 (98% [95% CI : 95-100]) [1 discontinuation, 1 LTFU] SVR12, by ITT, n/N (%) EXPEDITION-4 Gane E. NEJM 2017; 377:1448-55
EXPEDITION-4 Study: glecaprevir/pibrentasvir in patients with renal impairment Adverse events and laboratory abnormalities, % GLE/PIB , N = 104 Any adverse event Serious adverse event related to study drug Adverse event leading to discontinuation Death Adverse events in > 10% of patients, % Pruritus Fatigue Nausea Laboratory abnormalities, % Hemoglobin grade 3 (6.5-8 g/dL) AST grade 2 (> 3 x ULN) ALT grade 2 (> 3 x ULN) Total bilirubin grade 3 ( > 3 x ULN) 71 24 0 4 * 1 ** 20 14 12 5 0 0 1 * Diarrhea ; pruritus ; pulmonary edema, hypertensive cardiomyopathy with congestive failure ; hypertensive crisis ** Serious adverse event of cerebral hemorrhage, not related to study drug, at post-treatment W2 EXPEDITION-4 Gane E. NEJM 2017; 377:1448-55
EXPEDITION-4 Study: glecaprevir/pibrentasvir in patients with renal impairment Summary GLE/PIB (300 mg/120 mg QD) achieved high efficacy in patients with stage 4 or 5 chronic kidney disease and HCV infection 98% ITT SVR12rate across all major HCV genotypes No virologic failures GLE/PIB was well tolerated with a favorable safety profile in this difficult-to-treat population: No drug-related serious adverse event No grade 2 laboratory abnormalities in ALT or AST EXPEDITION-4 Gane E. NEJM 2017; 377:1448-55