Study on Ombitasvir/Paritaprevir/Ritonavir for HCV in HIV Coinfected Patients
Investigating the efficacy of ombitasvir/paritaprevir/ritonavir combined with dasabuvir and ribavirin in treating HCV in HIV-co-infected patients. The study includes baseline characteristics, treatment regimens, primary efficacy endpoints, and virologic outcomes of patients. Results show promising SVR rates, with considerations for prior treatments and mutations. Published in reputable journals by Sulkowski MS and Wyles D.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
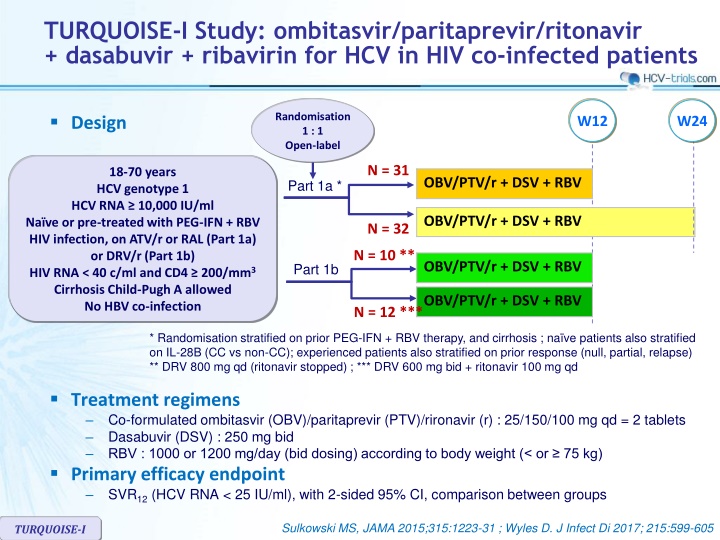
TURQUOISE-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV in HIV co-infected patients Design Randomisation 1 : 1 Open-label W12 W24 N = 31 18-70 years HCV genotype 1 HCV RNA 10,000 IU/ml Na ve or pre-treated with PEG-IFN + RBV HIV infection, on ATV/r or RAL (Part 1a) or DRV/r (Part 1b) HIV RNA < 40 c/ml and CD4 200/mm3 Cirrhosis Child-Pugh A allowed No HBV co-infection OBV/PTV/r + DSV + RBV Part 1a * OBV/PTV/r + DSV + RBV N = 32 N = 10 ** OBV/PTV/r + DSV + RBV Part 1b OBV/PTV/r + DSV + RBV N = 12 *** * Randomisation stratified on prior PEG-IFN + RBV therapy, and cirrhosis ; na ve patients also stratified on IL-28B (CC vs non-CC); experienced patients also stratified on prior response (null, partial, relapse) ** DRV 800 mg qd (ritonavir stopped) ; *** DRV 600 mg bid + ritonavir 100 mg qd Treatment regimens Co-formulated ombitasvir (OBV)/paritaprevir (PTV)/rironavir (r) : 25/150/100 mg qd = 2 tablets Dasabuvir (DSV) : 250 mg bid RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or 75 kg) Primary efficacy endpoint SVR12(HCV RNA < 25 IU/ml), with 2-sided 95% CI, comparison between groups Sulkowski MS, JAMA 2015;315:1223-31 ; Wyles D. J Infect Di 2017; 215:599-605 TURQUOISE-I
TURQUOISE-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV in HIV co-infected patients Baseline characteristics and patient disposition (Part 1a) 12 weeks N = 31 50.9 6% 26.4 87% / 13% 84 % 6.54 0.57 52 / 16 / 13 / 19 11 (35%) 5 5 1 633 52% / 48% 1 (withdrew consent) 24 weeks N = 32 50.9 9% 27.2 91% / 9% 78 % 6.60 0.78 63 / 16 / 3 / 19 10 (31%) 5 2 3 625 38% / 63% 1 (virologic breakthrough) Mean age, years Female Body mass index, mean Genotype : 1a / 1b IL28B non-CC genotype HCV RNA log10IU/ml, mean (SD) Fibrosis score F0-F1 / F2 / F3 / F4 (%) Prior treatment with PEG-IFN + RBV, N (%) Null response Partial response Relapse CD4/mm3, mean ARV regimen : ATV/r / RAL Discontinued treatment, N Sulkowski MS, JAMA 2015;315:1223-31 TURQUOISE-I
TURQUOISE-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV in HIV co-infected patients Virologic Outcome (Part 1a) 12 weeks N = 31 94% (95% CI: 79 - 98) 2 1 consent withdrawn 1 relapse Relapse NS3 : D168V NS5A : M28T NS5B : S556G 24 weeks N = 32 SVR12(HCV RNA < 25 IU/ml) 91 % (95% CI : 76 - 97) 3 1 virologic breakthrough 2 re-infection Breakthrough NS3 : R155K NS5A : Q30R NS5B : S556G Failure*, N Reasons Mutations detected Prior PEG-IFN + RBV therapy in failures Fibrosis stage in failures Na ve = 1, Null response = 1 Na ve = 2, Null response = 1 F3 / F4 F4 / F0-F1 / F0-F1 * All 5 = genotype 1a No HIV RNA failure (3 blips in W12-group and 5 blips in W24-group) Decrease in absolute but not relative CD4 cell counts Sulkowski MS, JAMA 2015;315:1223-31 TURQUOISE-I
TURQUOISE-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV in HIV co-infected patients Adverse events, Part 1 a, n (%) 12 weeks, N = 31 0 5 0 24 weeks, N = 32 0 6 0 AE leading to treatment discontinuation AE leading to RBV dose reduction Serious adverse event AE occurring in > 10% in either group Fatigue Insomnia Nausea Headache Upper respiratory tract infection Pruritus Cough Ocular icterus Diarrhea Hyperbilrubinemia ALT > 5 x ULN / AST > 5 x ULN Total bilirubin > 3 x ULN 58% 16% 16% 19% 13% 19% 7% 16% 3% 13% 0 / 0 35% 38% 22% 19% 13% 16% 6% 16% 3% 13% 3% 0 / 1 19% Sulkowski MS, JAMA 2015;315:1223-31 TURQUOISE-I
TURQUOISE-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV in HIV co-infected patients Baseline characteristics and SVR12 (Part 1b) OBV/PTV/r + DSV + RBV DRV qd, n = 10 OBV/PTV/r + DSV + RBV DRV bid, n = 12 Median age, years 56 53 Female, % 20 25 Body mass index, median 26 26 Genotype 1a, % 90 50 IL28B non-CC genotype, % 100 83 Cirrhosis, % 0 25 Prior treatment with PEG-IFN + RBV, % 30 0 DRV as first PI, % 30 33 CD4/mm3, median 656 612 SVR12 100% 100% HIV RNA blips : 2/10 DRV qd and 3/12 DRV bid Wyles D. Journal Infect Dis 2017 (ePub ahead of print) TURQUOISE-I
TURQUOISE-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV in HIV co-infected patients Pharmacokinetic parameters of DRV qd and bid (Part 1b) OBV/PTV/r + DSV + RBV DRV qd, n = 10 0.924 OBV/PTV/r + DSV + RBV DRV bid, n = 12 0.921 Cmax AUC 0.833 0.876 Ctrough 0.643 0.730 Values are the least square mean ratios (90% confidence intervals) for DRV pharmacokinetic parameters with and without OBV/PTV/r + DSV (DRV + OBV/PTV/r + DSV vs DRV alone) Wyles D. J Infect Di 2017; 215:599-605 TURQUOISE-I
TURQUOISE-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV in HIV co-infected patients Adverse events, Part 1 b, n OBV/PTV/r + DSV + RBV DRV qd, n = 10 OBV/PTV/r + DSV + RBV DRV bid, n = 12 AE leading to treatment discontinuation 0 0 1 (colitis and dehydration, not related to study drugs) Serious adverse event 0 AE occurring in > 15% in either group Fatigue 4 4 Hemoglobin decreased 1 4 Irritability 3 2 Nausea 2 2 ALT > 5 x ULN / AST > 5 x ULN 0 / 0 0 / 0 Total bilirubin > 3 x ULN 0 0 Hemoglobin 8-10 g/dl, < 8 g/dl 1 / 0 3 / 1 RBV dose reduction, n = 9 (decrease hemoglobin levels, n = 4 ; anemia, n = 3 ; fatigue, n = 2) Wyles D. J Infect Di 2017; 215:599-605 TURQUOISE-I
TURQUOISE-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV in HIV co-infected patients Summary In Part 1a of this open-label, randomised uncontrolled study, treatment with the all-oral, IFN-free 3D plus ribavirin regimen resulted in high SVR12rates among patients co-infected with HCV genotype 1 and HIV-1 whether treated for 12 or 24 weeks No discontinuation for AE The 2 failures (1 virologic breakthrough and 1 relapse) were observed in patients with genotype 1a and F4 fibrosis Limitations Small sample size ARV therapy limited to ATV/r- or RAL-containing regimens Role of RBV not addressed In Part 1b, HCV genotype 1 HIV-1 coinfected patients on stable DRV-containing ART achieved 100% SVR12while maintaining plasma HIV-1 RNA suppression. Despite DRV Ctroughdecrease, episodes of intermittent HIV-1 viremia were infrequent Sulkowski MS, JAMA 2015;315:1223-31 ; Wyles D. J Infect Di 2017; 215:599-605 TURQUOISE-I








