Study on Sofosbuvir and Ribavirin in HCV-HIV Co-infection
"Explore the PHOTON-1 Study focusing on Sofosbuvir and Ribavirin treatment for HCV-HIV co-infection. The non-randomized, open-label study analyzed treatment outcomes, patient characteristics, SVR12 rates, multivariate analysis, adverse events, and more in individuals with chronic HCV and HIV. Discover the results and factors associated with treatment success in this unique population."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
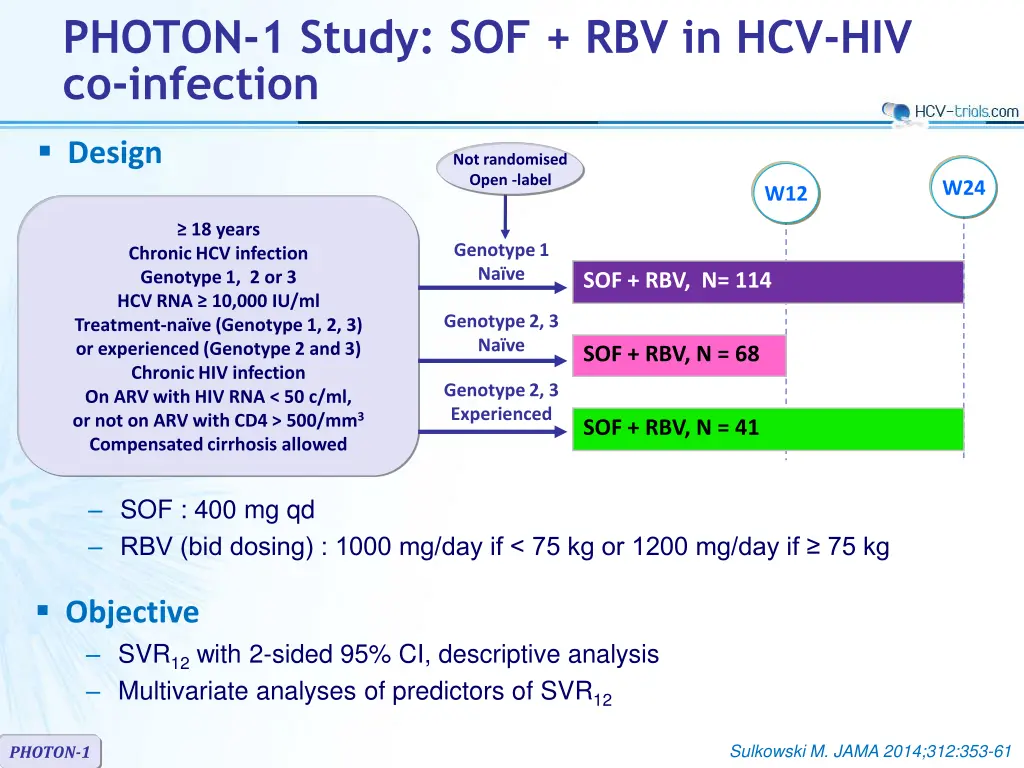
PHOTON-1 Study: SOF + RBV in HCV-HIV co-infection Design Not randomised Open -label W24 W12 18 years Genotype 1 Na ve Chronic HCV infection Genotype 1, 2 or 3 HCV RNA 10,000 IU/ml Treatment-na ve (Genotype 1, 2, 3) or experienced (Genotype 2 and 3) Chronic HIV infection On ARV with HIV RNA < 50 c/ml, or not on ARV with CD4 > 500/mm3 Compensated cirrhosis allowed SOF + RBV, N= 114 Genotype 2, 3 Na ve SOF + RBV, N = 68 Genotype 2, 3 Experienced SOF + RBV, N = 41 SOF : 400 mg qd RBV (bid dosing) : 1000 mg/day if < 75 kg or 1200 mg/day if 75 kg Objective SVR12with 2-sided 95% CI, descriptive analysis Multivariate analyses of predictors of SVR12 Sulkowski M. JAMA 2014;312:353-61 PHOTON-1
PHOTON-1 Study: SOF + RBV in HCV-HIV co-infection Baseline characteristics and patient disposition Genotype 1 na ve N = 115 Genotype 2, 3 na ve N = 68 Genotype 2, 3 experienced N = 41 Mean age, years 48 49 54 Female 18% 19% 10% Race : white/black 61% / 33% 79% / 12% 78% / 17% Body mass index, mean 27.3 27.4 27.3 HCV genotype 1a: 79% / 1b: 21% 2: 38% / 3: 62% 2: 59% / 3 : 41% IL28B CC genotype 26.5% 36.8% 48.8% HCV RNA log10IU/ml, mean (SD) Cirrhosis 6.6 0.83 6.3 0.60 6.5 0.69 4.4% 10.3% 24.4% CD4/mm3, median 581 562 579 On ARV 98.2% 89.7% 95.1% ARV (%) : EFV ; ATV/r ; DRV/r ; RAL ; RPV 38 ; 21 ; 13 ; 19 ; 6 33 ; 12 ; 28 ; 13 ; 8 41 ; 21 ; 5 ; 18 ; 5 Discontinued treatment, N 11 (3 AE, 1 Failure) 6 (3 AE) 1 (1 AE) Returned for post-treatment W12 visit 113 64 40 Sulkowski M. JAMA 2014;312:353-61 PHOTON-1
PHOTON-1 Study: SOF + RBV in HCV-HIV co-infection SVR12 (HCV RNA < 25 IU/ml), % (95% CI) Genotype 1 Na ve Genotype 2 and 3 Na ve Genotype 2 and 3 Experienced % 92.7 100 (80.1-98.5) 94 92 88 76.3 75 82 (67.4-83.8) (63-84.7) 75 67 54 50 25 N 114 90 24 68 26 42 41 24 17 0 GT2 GT3 GT2 GT3 All 1a 1b All All Sub-genotype Sulkowski M. JAMA 2014;312:353-61 PHOTON-1
PHOTON-1 Study: SOF + RBV in HCV-HIV co-infection Multivariate analysis of factors associated with SVR12in genotype 1 OR (95% CI) p Non black race 2.87 (1.01 - 8.20) 0.049 Genotype 1a 3.42 (1.15 - 10.16) 0.03 Completion of the 24 weeks of therapy 17.54 (3.77 - 83.33) < 0.001 Virologic failures Genotype 1 na ve N = 115 1 Non adherence Genotype 2, 3 na ve N = 68 1 (genotype 2) Non adherence All genotype 3 11/61 (18%) 1/6 (17%) Genotype 2, 3 experienced N = 41 Virologic breakthrough 0 Relapse In treatment completers In non-completers Mutations at relapse (deep sequencing) S282T or V321A L159F 19/103 (18%) 6/10 (60%) 1/40 (2.5%) 1/1 (100%) None 2 None 2 (genotype 3a) Sulkowski M. JAMA 2014;312:353-61 PHOTON-1
PHOTON-1 Study: SOF + RBV in HCV-HIV co-infection Adverse events, n (%) Genotype 1 na ve N = 115 3 8 (7%) Genotype 2, 3 na ve N = 68 3 5 (7%) Genotype 2, 3 experienced N = 41 1 1 (2%) AE leading to treatment discontinuation Serious adverse event AE occurring in 10% in either group Fatigue Insomnia Nausea Headache Irritability Cough Upper respiratory tract infection Diarrhea Dizziness Anemia Decreased hemoglobin < 10g/dl ; < 8.5 g/dl 36% 13% 16% 14% 12% 12% 11% 11% 6% 11% 35% 21% 18% 13% 10% 6% 12% 9% 1.5% 9% 46% 20% 15% 12% 5% 10% 12% 12% 12% 7% 12% / 0 19% / 2% 10% / 1.5% Sulkowski M. JAMA 2014;312:353-61 PHOTON-1
PHOTON-1 Study: SOF + RBV in HCV-HIV co-infection Summary In this open-label, non-randomised, uncontrolled study, patients with HCV who were co-infected with HIV had high rates of SVR12with the oral, IFN-free, combination of SOF + RBV After 24 weeks of therapy for genotype 1 treatment-na ve and genotype 2 and 3 treatment-experienced After 12 weeks of therapy for genotype 2 treatment-na ve Low rate of discontinuation for adverse events, however somewhat higher than in HCV monoinfected patients Confirmation of the high barrier to resistance of SOF (no resistance mutations at failure) Limitations Underrepresentation of women and patients with cirrhosis Absence of a control group Sulkowski M. JAMA 2014;312:353-61 PHOTON-1