Surface Ozone Concentration in Beijing: VOC Impact
Explore the impact of volatile organic compounds (VOCs) from various sources on surface ozone concentration during summer in Beijing, China. The study delves into different VOC sources, ambient concentrations, source profiles, and contributions, along with a model analyzing pure chemistry reactions. Discover the intricate relationship between VOCs and surface ozone levels to understand air quality challenges in urban environments.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Impact of VOCs from different sources on surface ozone concentration in summer in Beijing, China Hang Qu
Introduction Method Result
Introduction VOC August 2013 Vehicles Gasoline exhaust Gasoline evaporation LPG Diesel exhaust Industry Painting Petrochemical industry Biogenic 120 100 80 Ozone conc. [ppb] 60 40 20 0 0:00 6:00 12:00 Local Time 18:00 0:00
Method Ambient VOC concentration VOC source profiles and source apportionment Model
Ambient conc. of VOC Mean [ g/m3] Species i-Pentane 11.84 Toluene 11.14 m,p-xylene 8.54 n-butane 6.36 propane 6.24 acetylene 5.64 benzene 5.43 i-butane 5.36 ethylene 5.18 ethane 4.47 Song et al, 2008
Source profile and contributions Gasoline exhaust 20% VOC Source Contribution 10% Gasoline exhaust 39.7% 0% Gasoline evaporation 11.8% LPG 20% LPG 11.0% 10% 0% Diesel 3.2% Gasoline evaporation 20% Natural Gas 4.6% 10% Petrochemical 19.9% 0% Painting 4.7% Petrochemical 20% Biogenic Song et al, 2008 1.6% 10% 0% Acetylene 1-Pentene t-2-Butene n-Pentane Limonene n-Hexane i-Butane a-Pinene c-2-Butene n-Decane Ethylbenzene Isoprene MTBE Proplane t-2-Pentene Benzene Toluene 2M-Propene Ethylene 1-Butene Ethane c-2-Pentene n-Butane i-Pentane o-Xylene Propylene b-Pinene m,p-Xylene 2M-1-Butene 3M-1-Butene 2M-2-Butene
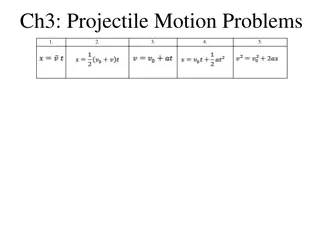
Model-pure chemistry Environment 298K, 1atm, noon in summer, 40 N 1800 ppb of Methane, 30 ppb of NOX Tracers 10 most abundant VOC and chemicals/radicals in their reaction pathway Reactions Full oxidation pathway for C2H6, C2H4, C2H2, C3H8 General oxidation pathway for hydrocarbon with 4 & 5 carbon atoms General oxidation pathway for Toluene and Xylene
Photolysis rate ? = ? ? ? ? ? ? ?? Kinetic constant Latest result from IUPAC F: actinic flux of zenith angle ~20 ?: absorption cross-section ?: quantum yield
Results Gasoline exhaust Gasoline evaporation 60.0% 20% 40.0% 10% 20.0% 0.0% 0% -100% -50% 0% 50% 100% -100% -50% 0% 50% 100% -20.0% -10% -40.0% -60.0% -20% ozone conc. Petrochemical voc conc. ozone conc. voc conc. LPG 20% 30% 20% 10% 10% 0% 0% -100% -50% 0% 50% 100% -100% -50% 0% 50% 100% -10% -10% -20% -20% -30% ozone conc. voc conc. ozone conc. voc conc.
Beijing NOX source Agriculture Budget(Mg/year) Contribution 0.0 0% Industry 130669.7 39.0% Power 61197.3 18.3% Residential 16756.7 5.0% Transportation 126290.9 37.7% MEIC 20% 1% 0% 0% -1% -20% -2% -40% -3% -60% -4% -80% -5% -100% -6% 0 20 40 60 15 20 25 30 35 40 45 50 55 60 NOx conc. [ppb] NOx conc. [ppb] ozone conc. ozone conc.
30% 0% -67% -10% -64% 20% -20% -61% 10% -30% -58% Petrochemical Emission Ozone conc. Gasoline Emission -40% -55% 0% -50% -52% -10% -60% -50% -70% -47% -20% -80% -44% -30% -90% -41% -60% -30% 0% 30% 60% VOC conc. -100% -38% -32% -29% -26% -23% gasoline exhaust gasoline evaporation Ozone petrochemical LPG
Conclusion With decreasing emission of 10% for gasoline exhaust, gasoline evaporation, petrochemical process and LPG will decrease the ozone concentration for 2.3%, 0.8%, 1.6%, 0.9% respectively. Decreasing petrochemical source is most effective while decreasing the same amount of VOC emitted