Synthesis Goals and Crystal Growth Techniques
This content delves into the goals of synthesis, such as preparing new compounds through exploratory synthesis and known compounds with controlled purity. It also explores crystal growth techniques for metals, ceramics, semiconductors, and nonmetals, highlighting methods like solid-state growth and vapor-state growth. Various strategies for crystal growth, including sublimation and gas phase reactions, are discussed in detail along with examples and processes to achieve single crystals.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
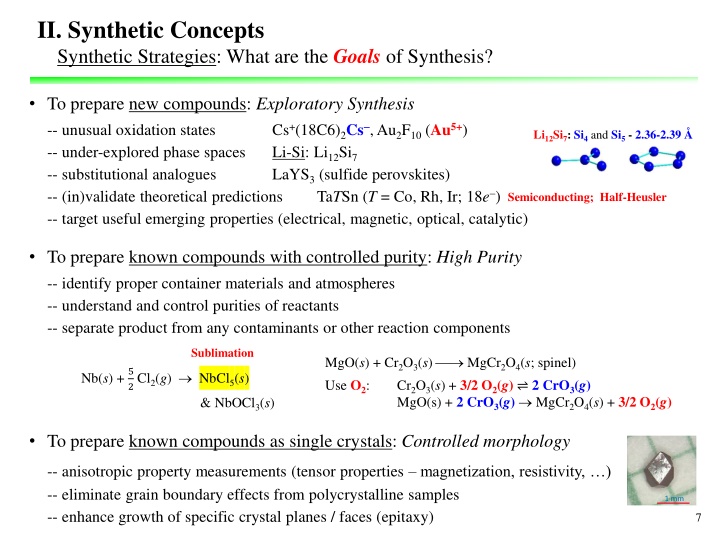
II. Synthetic Concepts Synthetic Strategies: What are the Goals of Synthesis? To prepare new compounds: Exploratory Synthesis -- unusual oxidation states -- under-explored phase spaces -- substitutional analogues -- (in)validate theoretical predictions -- target useful emerging properties (electrical, magnetic, optical, catalytic) Cs+(18C6)2Cs , Au2F10 (Au5+) Li-Si: Li12Si7 LaYS3 (sulfide perovskites) TaTSn (T = Co, Rh, Ir; 18e ) Li12Si7: Si4and Si5 - 2.36-2.39 Semiconducting; Half-Heusler To prepare known compounds with controlled purity: High Purity -- identify proper container materials and atmospheres -- understand and control purities of reactants -- separate product from any contaminants or other reaction components Sublimation MgO(s) + Cr2O3(s) MgCr2O4(s; spinel) Use O2: Cr2O3(s) + 3/2 O2(g) 2 CrO3(g) MgO(s) + 2 CrO3(g) MgCr2O4(s) + 3/2 O2(g) Nb(s) + 5 2 Cl2(g) NbCl5(s) & NbOCl3(s) To prepare known compounds as single crystals: Controlled morphology -- anisotropic property measurements (tensor properties magnetization, resistivity, ) -- eliminate grain boundary effects from polycrystalline samples -- enhance growth of specific crystal planes / faces (epitaxy) 7
II. Synthetic Concepts Synthetic Strategies: Crystal Growth Techniques Metals, Ceramics (Oxides), Pnictide and Chalcogenide Semiconductors Solid-State Growth (Metallurgical Processes) o No melting o Grains grow at the expense of smaller neighboring particles - Incomplete numerous grain boundaries and pores - Unreliable sample morphologies and defects vary o Micron-sized crystals Annealing Sintering - Hot Pressing Zone Heating 2 3?? ? ~ Time Powder Particles Intermediate Stage Final Product Sintering and Additive Manufacturing , M. Saline, Industrial Heating, 2019 8 B.R. Pamplin, Crystal Growth, 2nd Ed. Pergamon Press 1980.
II. Synthetic Concepts Synthetic Strategies: Crystal Growth Techniques Vapor-State Growth Nonmetals, Semiconductors (optoelectronics, etc.) o Control supersaturation by T gradients o Epitaxial Growth Homoepitaxy - orientation dependence - defect propagation Heteroepitaxy - lattice and thermal expansion matching - orientation relationships - defect generation Sublimation (Purification Method) - Vacuum Evaporation - Molecular Beam Epitaxy (MBE) Gas Transport (needs transport agent) Gas Phase Reactions - Chemical Vapor Deposition (CVD) - Vapor Phase Epitaxy (VPE) MOCVD: Flux of Molecules Impinging on a Surface Heating Zone Exhaust Inlet # Molecules Area Time ? MW ? Substrate EXAMPLE:Ga(C2H5)3(g) + AsH3(g) GaAs(s) + 3 C2H6(g) EXAMPLE:Ga(C2H5)3(g) + AsH3(g) 9 B.R. Pamplin, Crystal Growth, 2nd Ed. Pergamon Press 1980.
II. Synthetic Concepts Synthetic Strategies: Crystal Growth Techniques Melt Growth Metals, Semiconductors, Salts, some Organics o Congruently melting substances o Manageable vapor pressure at melting point o Seed crystal reduces supercooling and nucleation problems Freezing (normal, directional) Zone Melting - Float Zone Melting Crystal Pulling (seed crystal) Flame Fusion - Plasma Melting Example: Growth of Mn2O7(s) on X-ray diffractometer (Z. Anorg. Allg. Chem. 1988, 558, 7) Czochralski Technique Bridgman-Stockbarger Technique T Gradient Move sample (ampoule) Move furnace Seed Melt Melt Crystal Rotation: inhibits tendency to grow along specific orientations to produce facets. Graphite container Cu crystal Furnace 10 B.R. Pamplin, Crystal Growth, 2nd Ed. Pergamon Press 1980.
II. Synthetic Concepts Synthetic Strategies: Crystal Growth Techniques Solution Growth Solute crystallizes (precipitates) below its melting point Gel Growth Solvent Growth - Solvothermal (Hydrothermal) - Supercritical Fluids Liquid Phase Epitaxy Flux Growth o May achieve supersaturation (rapid growth) Solution Solid + Saturated Solution - Supercooling - Solvent extraction o Possible inclusion of solvent in crystal A flux-grown Yb-Ag-Ge crystal (Ames Laboratory) CuSO4 5 H2O crystals from saturated solution via slow solvent evaporation. The Power of One: Single Crystals Provide Clarity , Ames Laboratory, Jan 22, 2020 11 B.R. Pamplin, Crystal Growth, 2nd Ed. Pergamon Press 1980.
II. Synthetic Concepts Synthetic Strategies: Examples Gaseous Components Can enhance thermodynamic driving forces and reaction kinetics Metathesis (Exchange) reactions: (often redox processes) MnO2(s) + CO(g) MnO(s) + CO2(g) ? > 0 Often entropy-driven Hydrogen reduction: x Cu(s) + 6 MoS2(s) + 4 H2(g) CuxMo6S8(s) + 4 H2S(g) Chevrel Phases : Superconductors; HDS Catalysts Pt catalyst 1000 C Combination reactions: Gd(s) + H2(g) GdH2(s) (embrittlement, powder) La2O3(s) + xCO2(g) La2O3 x(CO3)x 1000 C ? < 0, ? < 0 Enthalpy-driven Decomposition reactions: Y2O3(s) + 4 BaCO3 + 6 CuO(s) 2 YBa2Cu3O7 (s) + 4 CO2(g) Superconducting Air or O2 Vapor Transport equilibria: Transport agents = I2, O2, H2O, HCl, ... FeSi(s) + 7/2 I2(g) FeI3(g) + SiI4(g) TiO2(s) + 4 HCl(g) TiCl4(g) + 2 H2O(g) ?Low and ?High Zones Solid grows where ? ? is lower 12
II. Synthetic Concepts Synthetic Strategies: Examples High Pressure Creates more contact and reactive interfaces between particles Enhances atomic diffusion via internal stresses Provides energy via p V work 1 atm = 0.1 MPa 1 atm 1 bar 1 atm = 760 torr 1 atm = 0.1 MPa : 1 kJ 1 GPa cm3 100-200 GPa = 1-2 Mbar High hydrostatic pressure on reaction mixture(diamond anvil cell, ) Unprecedented Compounds: H3S (155 GPa) Na2He (300 GPa) NaCl3 (60 GPa) Superconducting KClO4 decomp. High pressure of reactive gas O2 Decomposition of KClO3, KO2, ... F2 Need Teflon or Monel autoclaves BaPrF6 ; [O2]+[AuF6] Ag2NiO2 (550 C; 65 MPa O2): [Ag2]+[NiO2] : AFM Metal Ag2NiO2 (550 C; 65 MPa O2) LaCuO3 (1400 C; 5 GPa O2) La2CuO4 + CuO/Cu2O LaCuO3 (1400 C; 5 GPa O2) (800 C; 1 atm) High solvent pressures under solvothermal conditions (autoclave) Hydrothermal conditions (Geochemistry, Mineralogy) oxides, hydroxides Ammonothermal conditions nitrides, amides, imides (Near) Supercritical Solvents (polar protic or aprotic; nonpolar) P.C. Burnley, The Diamond Anvil Cell (DAC) 13 P. Hagenmuller, Ed., Preparative Methods in Solid State Chemistry, Academic Press: 1972.
II. Synthetic Concepts Synthetic Strategies: Examples Liquid-Phase Components Can increase contact areas between reactants Can increase atom mobilities and reaction rates Can dissolve products Fusion of one or more components can prepare condensed phases neat Gd(s) + 2 GdI3(l) 3 GdI2(s) Synproportionation Useful if product melts congruently As liquid is depleted, diffusion problems inherent in solid-solid reactions can recur Solid-State Metathesis: Exothermic reaction, generated heat can accelerate reaction or enhance separation of products by producing liquids (or gases): UF4(s) + 2 Mg(s) U(s) + 2 MgF2(s) U(l) + 2 MgF2(s, l) 1408 K 0 ?298 = ???,??? ? 1536 K Lattice energy of product (MgF2) provides driving force Achieve final state control by heating reactants or including product (absorbs some sensible heat ) Liquid products can be separated (density differences) 14
II. Synthetic Concepts Synthetic Strategies: Examples Solutions Can lower reaction temperatures compared to solid-state reactions Can increase atom mobilities and reaction rates Reactions can be too fast and give finely divided or amorphous material Liquid Solutions eutectic mixture of 2 salts lowers freezing point of each salt LiCl / RbCl mixtures used to prepare complex sulfides and sulfosalts. 718 C 605 C Liquid Liquid + Solid Liquid + Solid Li2CO3 + 5 Fe2O3 2 LiFe5O8 + CO2(g) Reactive sintering gives incomplete conversion Li2SO4 / Na2SO4 flux at 800 C dissolves Li2CO3; Remove with water 318 C 308 C Solid LiCl RbCl Solid Solutions intimate mixtures of elements Solid Solutions intimate mixtures of elements O2; T > 800 C (Time; Temperature) 2 CaCO3 + 3 MnCO3 CaO + MnO2 x + CaMnO3 / CaMn2O4 2 CaCO3 + 3 MnCO3 T < 900 C 5 Ca0.4Mn0.6CO3 Ca2Mn3O8(s) + 5 CO2(g) 5 Ca0.4Mn0.6CO3 Aqueous Precipitate Stable at T < 890 C under 1 atm O2 15
II. Synthetic Concepts Synthetic Strategies: Examples Other Methods Chimie Douce ( Soft Chemistry ): Avoid high temperatures (energy and time intensive) Utilize strategies developed by living organisms, e.g. biomineralization Intercalation and De-intercalation Reactions n-BuLi / Ethane HCl(aq) WO3(s) LixWO3(s) Sol-Gel Process: inorganic polymerization reactions from solution-based precursors CuxMo6S8(s) Mo6S8(s) Microwave-aided Synthesis: 0.01 1.00m, 0.9 or 2.45 GHz Heating is ... Direct wave radiation interacts with reaction components Selective specific reactants interact differently with waves Volumetric uniform heating throughout sample Rapid fast reaction times; immediate quenching Temperature Profiles Microwaves Conventional (Heat Bath) Oxides, Chalcogenides, Borides, Carbides, Nitrides, Silicides, ... Mechanochemistry (Ball-Milling): Mechanical forces via grinding Rapid, environmentally-friendly, solution-free, low temperatures Balls: Inorganics ( hard ) WC, ZrO2 Balls: Organics ( soft ) steel, agate, PMMA LiAlH4(s) + 2 LiH(s) Li3AlH6(s) 16
II. Synthetic Concepts Synthetic Strategies: Quality Criteria Concerns Incomplete conversion of reactants into products Separate by filtration, distillation, centrifugation, ... Unwanted side reactions Reaction with container, gas atmosphere, or adventitious impurities Metal source is really a metal hydride: Ba contains up to 20 atom % H Brass screen incorporates Cu into product ( Ta2AuS5 Ta2Cu0.8S6) Actions Minimize impurities Clean and dry container materials Clean gaseous reagents (odorants added to inert gases) Purify reactants (sublimation, distillation; ...) Sound quantitative analysis (bulk measurements) Diffraction (powder X-ray) Microscopy (SEM-EDS) Elemental analysis (Microprobe, ICP-MS) Spectroscopy (X-ray fluorescence, ESCA) 17
II. Synthetic Concepts Synthetic Strategies: Quality Criteria Some Historical Problems O -Tungsten: dendritic deposit, electrolysis of phosphate melt really(?) W3O W Ti Ti2S: prepared in graphite crucible really Ti2SC C S Sr Sr3Sn: semiconducting behavior really Sr3SnO O Sn In5S4: prepared using a Sn flux; could not be prepared pure in binary mixture really In4SnS4 [In In4]8+: 7 e s [Sn In4]8+: 8 e s 18