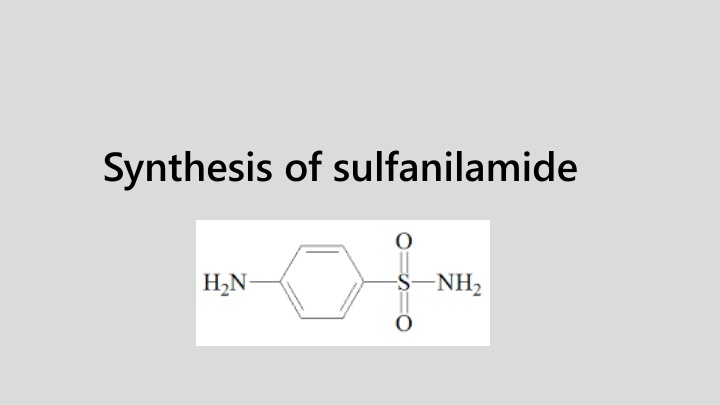
Synthesis of Sulfanilamide: Procedure and Steps
Discover the detailed synthesis process of sulfanilamide starting from acetanilide, including necessary precautions and steps to yield the product efficiently. Learn about the antibacterial properties and mechanism of sulfanilamide as an inhibitor of folic acid formation essential for bacterial growth.
Uploaded on | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Sulfanilamide or p-amino benzene sulfonamide was first synthesized in 1908. Sulfonamide has antibacterial properties. It acts by inhibiting the formation of folic acid that is necessary for bacterial growth.
Sulfanilamide would be synthesized through three steps, starting with acetanilide.
Before the first step, an important issue would take place, which is the generation of the electrophile as shown below:
Procedure Weight (2.5 gm) of acetanilide in dry clean beaker. Add enough volume (ml) of Chlorosulfonic acid drop by drop with heating (50 C0) until solid is dissolved. The product transferred to a beaker contain crushed ice. Note: caution is used in this step as unreacted chlorosulfonic acid produce acidic gases (Hcl) upon addition to water. White granules would result and collected via vacuum filtration. Press and drain the granules, then transfer it to another beaker. Add enough volume (ml) of liquid NH3 (25%) to the beaker and heat it for (10-15 minutes) (enough volume added until dissolving the product). Cool the product in an ice bath then vacuum filter it. Weight the dry product and calculate the percent % of yield.
