The Chemistry of Alkanes: Properties, Combustion, and Environmental Impact
The general formula and properties of alkanes, their combustion products' environmental damage, and methods to remove harmful substances. Explore the formation of free radicals, cracking of alkanes, and the impact on the ozone layer. Dive into the structure and physical properties of alkanes, including boiling points and isomerism. Learn about petrochemicals derived from alkanes and their varied uses across industries.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
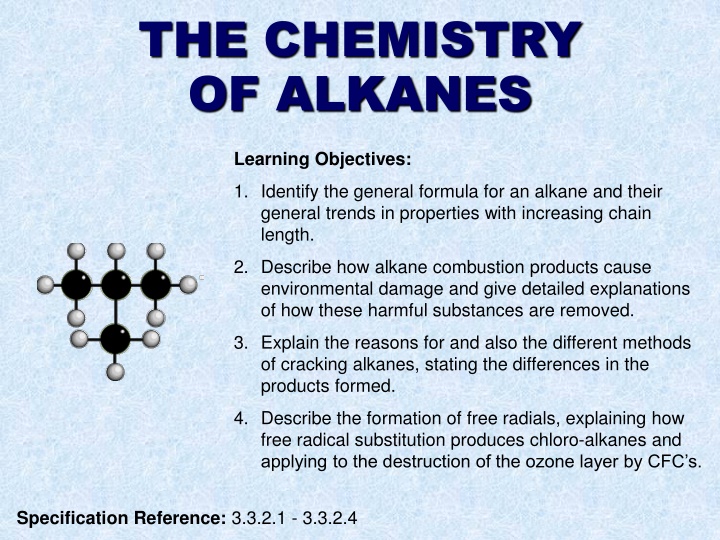
THE CHEMISTRY OF ALKANES Learning Objectives: 1. Identify the general formula for an alkane and their general trends in properties with increasing chain length. 2. Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed. 3. Explain the reasons for and also the different methods of cracking alkanes, stating the differences in the products formed. 4. Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s. Specification Reference: 3.3.2.1 - 3.3.2.4
ALKANES General members of a homologous series general formula is CnH2n+2 - for non-cyclic alkanes saturated hydrocarbons - all carbon-carbon bonding is single bonds are spaced tetrahedrally about carbon atoms. Isomerism the first example of structural isomerism occurs with C4H10 BUTANE 2-METHYLPROPANE Structural isomers have the SAME MOLECULAR FORMULA BUT DIFFERENT STRUCTURAL FORMULA They possess different physical properties such as boiling point, melting point and density LO1: Identify the general formula for an alkane and their general trends in properties with increasing chain length.
THE STRUCTURE OF ALKANES In ALKANES, the four sp3 orbitals of carbon repel each other into a TETRAHEDRAL arrangement with bond angles of 109.5 . Each sp3 orbital in carbon overlaps with the 1s orbital of a hydrogen atom to form a C-H bond. 109.5 LO1: Identify the general formula for an alkane and their general trends in properties with increasing chain length.
PHYSICAL PROPERTIES OF ALKANES Boiling point increases as they get more carbon atoms in their formula more atoms = greater induced dipole-dipole interactions greater intermolecular force = more energy to separate the molecules greater energy required = higher boiling point CH4 (-161 C) C2H6 (-88 C) C3H8 (-42 C) C4H10 (-0.5 C) difference gets less - mass increases by a smaller percentage Straight chains molecules have greater interaction than branched STRUCTURAL ISOMERS OF C5H12 HIGHEST BOILING POINT LOWESTBOILINGPOINT The greater the branching, the lower the boiling point LO1: Identify the general formula for an alkane and their general trends in properties with increasing chain length.
PETROCHEMICALS Boiling range / C molecule fraction C s per Name of Use(s) < 25 1 - 4 LPG (Liquefied Petroleum Gas) Calor Gas Camping Gas 40-100 4 - 12 GASOLINE Petrol 100-150 7 - 14 NAPHTHA Petrochemicals 150-200 11 - 15 KEROSINE Aviation Fuel 220-350 15 - 19 GAS OIL Central Heating Fuel > 350 20 - 30 LUBRICATING OIL Lubrication Oil > 400 30 - 40 FUEL OIL Power Station Fuel Ship Fuel > 400 40 - 50 WAX, GREASE Candles Grease for bearings > 400 > 50 BITUMEN Road surfaces Roofing LO1: Identify the general formula for an alkane and their general trends in properties with increasing chain length.
PHYSICAL PROPERTIES OF ALKANES Melting point general increase with molecular mass the trend is not as regular as that for boiling point. Solubility alkanes are non-polar so are immiscible with water they are soluble in most organic solvents. LO1: Identify the general formula for an alkane and their general trends in properties with increasing chain length.
CHEMICAL PROPERTIES OF ALKANES Introduction -fairly unreactive; (old family name, paraffin, meant little reactivity) - have relatively strong, almost NON-POLAR, SINGLE covalent bonds - they have no real sites that will encourage substances to attack them Combustion - make useful fuels - especially the lower members of the series - react with oxygen in an exothermic reaction complete combustion CH4(g) + 2O2(g) > CO2(g) + 2H2O(l) incomplete combustion CH4(g) + 1 O2(g) > CO(g) + 2H2O(l) BUT the greater the number of carbon atoms, the more energy produced the greater the amount of oxygen needed for complete combustion. Handy tip When balancing equations involving complete combustion, remember... every carbon in the original hydrocarbon gives one carbon dioxide and every two hydrogen atoms gives a water molecule. Put the numbers into the equation, count up the O s and H s on the RHS of the equation then balance the oxygen molecules on the LHS. LO1: Identify the general formula for an alkane and their general trends in properties with increasing chain length.
POLLUTION Processes involving combustion give rise to a variety of pollutants... power stations internal combustion engines SO2 emissions produce acid rain CO, NOxandunburnt hydrocarbons Removal SO2 react effluent gases with a suitable compound (e.g. CaO) = FLUE GAS DESULPHURISATION (NEUTRALISATION) CO and NOx pass exhaust gases through a catalytic converter LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
DEPLETION OF THE OZONE LAYER OXIDES OF NITROGEN NOx Oxides of nitrogen, NOx, formed during thunderstorms or by aircraft break down to give NO (nitrogen monoxide) which also catalyses the breakdown of ozone. nitrogen monoxide reacts with ozone O3 + NO > NO2 + O2 nitrogen monoxide is regenerated NO2 + O > O2 + NO LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
POLLUTANTS POLLUTANT GASES FROM INTERNAL COMBUSTION ENGINES Carbon monoxide CO Origin incomplete combustion of hydrocarbons in petrol because not enough oxygen was present Effect poisonous combines with haemoglobin in blood prevents oxygen being carried to cells Process C8H18(g) + 8 O2(g) > 8CO(g) + 9H2O(l) LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
POLLUTANTS POLLUTANT GASES FROM INTERNAL COMBUSTION ENGINES Oxides of nitrogen NOx - NO, N2O and NO2 Origin combination of atmospheric nitrogen and oxygen under high temperature Effect aids formation of photochemical smog which is irritating to eyes, nose, throat aids formation of low level ozone which affects plants and is irritating to eyes, nose and throat Process sunlight breaks oxides ozone is produced NO2 > NO + O O + O2 > O3 LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
POLLUTANTS POLLUTANT GASES FROM INTERNAL COMBUSTION ENGINES Unburnt hydrocarbons CxHy Origin hydrocarbons that have not undergone combustion Effect toxic and carcinogenic (cause cancer) LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
POLLUTANTS POLLUTANT FORMATION Nitrogen combines with oxygen N2(g) + O2(g) > 2NO(g) Nitrogen monoxide is oxidised 2NO(g) + O2(g) > 2NO2(g) Incomplete hydrocarbon combustion C8H18(g) + 8 O2(g) > 8CO(g) + 9H2O(l) LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
POLLUTANTS POLLUTANT REMOVAL Oxidation of carbon monoxide 2CO(g) + O2(g) > 2CO2(g) Removal of NO and CO 2CO(g) + 2NO(g) > N2(g) + 2CO2(g) Aiding complete hydrocarbon combustion C8H18(g) + 12 O2(g) > 8CO2(g) + 9H2O(l) LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
CATALYTIC CONVERTERS REMOVAL OF NOx and CO CO is converted to CO2 NOx are converted to N2 2NO(g) + 2CO(g) > N2(g) + 2CO2(g) LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
CATALYTIC CONVERTERS REMOVAL OF NOx and CO CO is converted to CO2 NOx are converted to N2 2NO(g) + 2CO(g) > N2(g) + 2CO2(g) Unburnt hydrocarbons converted to CO2 and H2O C8H18(g) + 12 O2(g) > 8CO2(g) + 9H2O(l) LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
CATALYTIC CONVERTERS REMOVAL OF NOx and CO CO is converted to CO2 NOx are converted to N2 2NO(g) + 2CO(g) > N2(g) + 2CO2(g) Unburnt hydrocarbons converted to CO2 and H2O C8H18(g) + 12 O2(g) > 8CO2(g) + 9H2O(l) Catalysts are rare metals - RHODIUM, PALLADIUM, PLATINUM Metals are finely divided for a greater surface area - this provides more active sites Leaded petrol must not pass through the catalyst as the lead deposits on the catalyst s surface and poisons it, thus blocking sites for reactions to take place. LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
CATALYTIC CONVERTERS STAGES OF OPERATION Adsorption Reaction Desorption LO2: Describe how alkane combustion products cause environmental damage and give detailed explanations of how these harmful substances are removed.
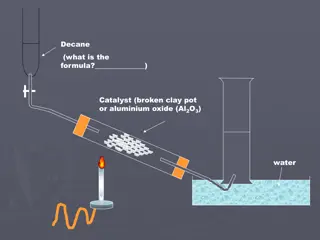
CRACKING Involves the breaking of C-C bonds in alkanes Converts heavy fractions into higher value products THERMAL CATALYTIC proceeds via a free radical mechanism proceeds via a carbocation (carbonium ion) mechanism THERMAL HIGH PRESSURE ... 7000 kPa HIGH TEMPERATURE ... 400 C to 900 C FREE RADICAL MECHANISM HOMOLYTIC FISSION PRODUCES MOSTLY ALKENES ... e.g. ETHENE for making polymers and ethanol PRODUCES HYDROGEN ... used in the Haber Process and in margarine manufacture Bonds can be broken anywhere in the molecule by C-C bond fission or C-H bond fission LO3: Explain the reasons for and also the different methods of cracking alkanes, stating the differences in the products formed.
CRACKING Involves the breaking of C-C bonds in alkanes Converts heavy fractions into higher value products THERMAL CATALYTIC proceeds via a free radical mechanism proceeds via a carbocation (carbonium ion) mechanism CATALYTIC SLIGHT PRESSURE HIGH TEMPERATURE ... 450 C ZEOLITE CATALYST CARBOCATION (IONIC) MECHANISM HETEROLYTIC FISSION PRODUCES BRANCHED AND CYCLIC ALKANES, AROMATIC HYDROCARBONS USED FOR MOTOR FUELS ZEOLITES are crystalline aluminosilicates; clay like substances LO3: Explain the reasons for and also the different methods of cracking alkanes, stating the differences in the products formed.
BREAKING COVALENT BONDS There are 3 ways to split the shared electron pair in an unsymmetrical covalent bond. UNEQUAL SPLITTING produces IONS known as HETEROLYSIS or HETEROLYTICFISSION EQUAL SPLITTING produces RADICALS known as HOMOLYSIS or HOMOLYTICFISSION If several bonds are present the weakest bond is usually broken first Energy to break bonds can come from a variety of energy sources - heat / light In the reaction between methane and chlorine either can be used, however... In the laboratory a source of UV light (or sunlight) is favoured. LO3: Explain the reasons for and also the different methods of cracking alkanes, stating the differences in the products formed.
FREE RADICALS TYPICAL PROPERTIES reactive species (atoms or groups) which possess an unpaired electron their reactivity is due to them wanting to pair up the single electron formed by homolytic fission (homolysis) of covalent bonds formed during the reaction between chlorine and methane formed during thermal cracking involved in the reactions taking place in the ozone layer LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
CHLORINATION OF METHANE Reagents chlorine and methane Conditions UV light or sunlight - heat is an alternative energy source CH4(g) + Cl2(g) > HCl(g) + CH3Cl(g) chloromethane CH3Cl(g) + Cl2(g) > HCl(g) + CH2Cl2(l) dichloromethane CH2Cl2(l) + Cl2(g) > HCl(g) + CHCl3(l) trichloromethane CHCl3(l) + Cl2(g) > HCl(g) + CCl4(l) tetrachloromethane Equation(s) Mixtures Mechanism free radicals are very reactive - they are trying to pair their electron with sufficient chlorine, every hydrogen will eventually be replaced. Mechanisms portray what chemists think is going on in the reaction, whereas an equation tells you the ratio of products and reactants. Chlorination of methane proceeds via FREE RADICAL SUBSTITUTION because the methane is attacked by free radicals resulting in hydrogen atoms being substituted by chlorine atoms. The process is a chain reaction. In the propagation step, one radical is produced for each one used LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
CHLORINATION OF METHANE Cl2 > 2Cl Initiation RADICALS CREATED The single dots represent UNPAIRED ELECTRONS During initiation, the WEAKEST BOND IS BROKEN as it requires less energy. There are three possible bonds in a mixture of alkanes and chlorine. Average bond enthalpy kJ mol-1 412 348 242 The Cl-Cl bond is broken in preference to the others as it is the weakest and requires requires less energy to separate the atoms. LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
CHLORINATION OF METHANE Cl + CH4 > CH3 + HCl Cl2 + CH3 > CH3Cl + Cl Propagation RADICALS USED and then RE-GENERATED Free radicals are very reactive because they want to pair up their single electron. They do this by abstracting a hydrogen atom from methane; a methyl radical is formed The methyl radical is also very reactive and attacks a chlorine molecule A chlorine radical is produced and the whole process can start over again LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
CHLORINATION OF METHANE Cl + Cl > Cl2 Cl + CH3 > CH3Cl CH3 + CH3 > C2H6 Termination RADICALS REMOVED Removing the reactive free radicals brings an end to the reaction. This is not very likely at the start of the reaction because of their low concentration. LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
CHLORINATION OF METHANE OVERVIEW Initiation Cl2 > 2Cl radicals created Propagation Cl + CH4 > CH3 + HCl Cl2 + CH3 > CH3Cl + Cl radicals used and then re-generated Termination Cl + Cl Cl + CH3 CH3 + CH3 > Cl2 > CH3Cl > C2H6 radicals removed Summary Due to lack of reactivity, alkanes need a very reactive species to persuade them to react Free radicals need to be formed by homolytic fission of covalent bonds This is done by shining UV light on the mixture (heat could be used) Chlorine radicals are produced because the Cl-Cl bond is the weakest You only need one chlorine radical to start things off With excess chlorine you get further substitution and a mixture of chlorinated products LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
CHLORINATION OF METHANE RADICALS PRODUCED Initiation Propagation RADICALS USED AND REGENERATED Termination RADICALS REMOVED LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
CHLORINATION OF METHANE Further propagation dichloromethane If excess chlorine is present, further substitution takes place The equations show the propagation steps for the formation of... Cl + CH3Cl > CH2Cl + HCl Cl2 + CH2Cl > CH2Cl2 + Cl Cl + CH2Cl2 > CHCl2 + HCl Cl2 + CHCl2 > CHCl3 + Cl trichloromethane > CCl3 + HCl > CCl4 + Cl tetrachloromethane Cl + CHCl3 Cl2 + CCl3 Mixtures Because of the many possible reactions there will be a mixture of products. Individual haloalkanes can be separated by fractional distillation. LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
DEPLETION OF THE OZONE LAYER Although ozone is a reactive and poisonous gas, it protects us from harmful UV radiation which would affect life on earth. UV radiation can cause skin cancer. LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
DEPLETION OF THE OZONE LAYER Although ozone is a reactive and poisonous gas, it protects us from harmful UV radiation which would affect life on earth. UV radiation can cause skin cancer. Ozone in the stratosphere breaks down naturally Ozone (trioxygen) can break up to give ordinary oxygen and an oxygen radical 2O3 > 3O2 O3 > O + O2 LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
DEPLETION OF THE OZONE LAYER Although ozone is a reactive and poisonous gas, it protects us from harmful UV radiation which would affect life on earth. UV radiation can cause skin cancer. Ozone in the stratosphere breaks down naturally Ozone (trioxygen) can break up to give ordinary oxygen and an oxygen radical Ultra violet light can supply the energy for the process. That is why the ozone layer is important as it protects us from the harmful rays. 2O3 > 3O2 O3 > O + O2 BUT breakdown is easier in the presence of chlorofluorocarbons (CFC's) LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.
DEPLETION OF THE OZONE LAYER EFFECT OF CFC S There is a series of complex reactions but the basic process is :- CCl2F2 > Cl + CClF2 CFC's break down in the presence of UV light to form chlorine radicals chlorine radicals react with ozone O3 + Cl > ClO + O2 ClO + O > O2 + Cl chlorine radicals are regenerated Overall chlorine radicals are not used up so a small amount of CFC's can destroy thousands of ozone molecules before the termination stage. LO4: Describe the formation of free radials, explaining how free radical substitution produces chloro-alkanes and applying to the destruction of the ozone layer by CFC s.