The Survival of Dissolved H2S: pH, Temperature, and Salinity Dependency
The survival of dissolved H2S is highly influenced by pH, temperature, and salinity. This dependency is illustrated by the chemical reactions involved, as shown in the provided figures. The chemistry of spring waters in sulfuric acid caves from various locations is also analyzed. Further oxidation pathways and industrial production processes of sulfuric acid are discussed. Biogenic sulfide corrosion and its impact on concrete structures due to bacterial activities are explored, emphasizing the conversion of H2S to H2SO4.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
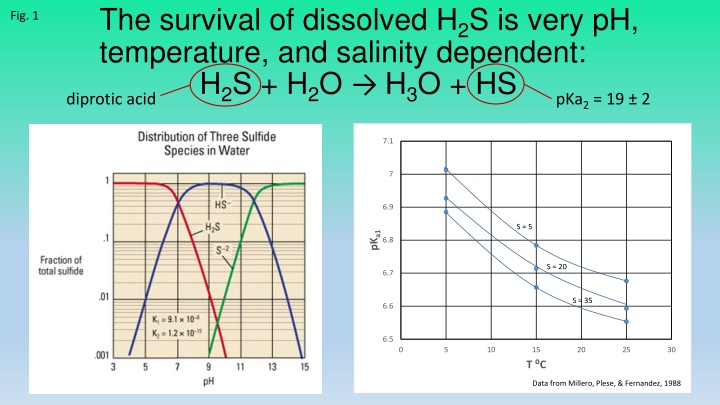
The survival of dissolved H2S is very pH, temperature, and salinity dependent: H2S + H2O H3O + HS diprotic acid Fig. 1 pKa2 = 19 2 7.1 7 6.9 S = 5 pKa1 6.8 S = 20 6.7 S = 35 6.6 6.5 0 5 10 15 20 25 30 T C Data from Millero, Plese, & Fernandez, 1988
Fig. 2 Chemistry of Spring Water of Active Sulfuric Acid Caves Chemistry of Spring Water of Active Sulfuric Acid Caves CAVE SPRING WATER PARAMETERS CAVE/COUNTRY Temperature C pH SO4 mg/L Cl mg/L H2S mg/L DO mg/L Cueva de Villa Luz, Mexico 28-30 6.61-7.3 920-940 782-814 100-500 ND-2.2 Frasassi Caves, Italy 13.1-13.7 6.9-7.4 240 658.7 22.2 -- Acquasanta Terme Caves, Italy 33.1-43.4 6.58-6.75 669-957 1697-3100 13.0-28.1 -- Montecchio Cave, Italy 31.3-36 6.86 1313 18.8 -- -- Parrano Cave, Italy 27 6.33 173 208 10 -- Serpents Cave, France 35.5-46.6 6.5 60-230 15-30 5 -- Aghia Paraskevi Caves, Greece 35-39.2 6.35-7.0 -- 19,467.5 101.6 -- Lower Kane Cave, USA 22.6-24 6.8-7.25 182-319 12.3-14.5 5-6 ND Fairy Cave, USA 49.6 6.39 1102 9706 1.65 0.48
Fig. 3 H2S + 2O2 2H2S(aq) + O2(aq) 2S + 2H2O H2SO4 Further oxidation yields SO3, S2O3, and finally SO4. Biotic oxidation enhances the rate by 3 or more orders of magnitude, but the outcome is the same. Sulfuric acid is the world s number one industrial chemical, and over 230 million metric tons were produced in 2012. It is produced in a 4-step process: S(s) + O2 SO2, 2SO2 + O2 2SO3, SO3 + H2O H2SO4 >200 C 2H2S(g) + 3O2(g) 2SO2 + 2H2O Or as an alternative sulfur source:
Fig. 4 pipe pipe A modern analog outside of speleogenesis: g gypsum rinds ypsum rinds Biogenic Sulfide Corrosion Internal corrosion of concrete wastewater pipes and tanks (man-made caves) due to anaerobic sulfate-reducing bacteria generating H2S, which is oxidized to sulfur in the condensate, which aerobic sulfur-oxidizing bacteria metabolize into H2SO4. Only occurs if the pipe or tank is partially full (air space above water is required) biofilms biofilms The acid converts CaCO3 into gypsum A process studied since the 1940s