Thermochemistry: Energy in Chemical Reactions
Dive into the world of thermochemistry to explore how energy plays a crucial role in chemical reactions. Learn about exothermic and endothermic processes, the interchangeability of kinetic and potential energy, and the concept of total energy in systems and surroundings.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Spring Semester Final Exam Review Thermochemistry
THERMOCHEMISTRY The study of heat released or required by chemical reactions Fuel is burnt to produce energy - combustion (e.g. when fossil fuels are burnt) CH4(g) + 2O2(g) CO2(g) + 2H2O(l) + energy
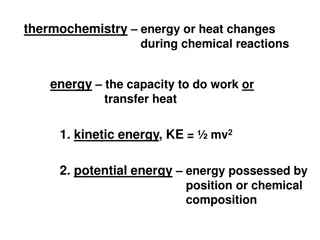
What is Energy? Energy Kinetic energy (KE) Potential energy (PE) Energy due to motion Stored energy
Total Energy = Kinetic Energy + Potential Energy E = KE + PE Temperature measures the average Kinetic energy & potential energy are interchangeable Heat is the total energy of a system: Kinetic energy + potential energy
Systems & Surroundings In thermodynamics, the world is divided into a system and its surroundings A system is the part of the world we want to study (e.g. a reaction mixture in a flask) The surroundings consist of everything else outside the system
EXOTHERMIC & ENDOTHERMIC REACTIONS Exothermic process: a change (e.g. a chemical reaction) that releases heat to the surroundings. A release of heat corresponds to a decrease in enthalpy Exothermic process: H < 0 (at constant pressure) Burning fossil fuels is an exothermic reaction
Endothermic process: a change (e.g. a chemical reaction) that requires (or absorbs) heat from the surroundings. An input of heat corresponds to an increase in enthalpy Endothermic process: H > 0 (at constant pressure) Photosynthesis is an endothermic reaction (requires energy input from sun)
exothermic endothermic exothermic endothermic endothermic
Heating Curves Animation Heating Curves Animation A plot of temperature vs. time that represents the process in which energy is added at a constant rate Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Gas - KE 140 120 100 80 60 Boiling - PE Temperature (oC) 40 20 Liquid - KE 0 Melting - PE -20 -40 -60 -80 -100 Solid - KE Time
A plot of temperature vs. time that represents the process in which energy is added at a constant rate
The standard enthalpy of reaction (H0 ) is the enthalpy of a reaction carried out at 1 atm. rxn aA + bB cC + dD a H0 (A) b H0 (B) H0rxn c H0 (C) d H0 (D) ] - [ + ] = [ + f f f f m H0 (reactants) f H0rxn n H0 (products) f = -
Example Problem Calculate the heat of combustion of methane, CH4 CH4(g) +2O2(g) CO2(g) + 2 H2O(g) H fCH4 (g) = -74.86 kJ/mol H fO2(g) = 0 kJ/mol H fCO2(g) = -393.5 kJ/mol H f H2O(g) = -241.8 kJ/mol pg. 316 2 mol(-241.8 kJ/mol) =-483.6 kJ Step #1: multiply the H f H2O(g) by 2 since there are two moles of water in the products . 15
Example Problem Calculate the heat of combustion of methane, CH4 CH4(g) +2O2(g) CO2(g) + 2 H2O(g) H fCH4 (g) = -74.86 kJ H fO2(g) H fCO2(g) = -393.5 kJ H fH2O(g) = -483.6 kJ = 0 kJ/ pg. 316 H f=[-393.5 kJ + (-483.6 kJ)]- [-74.86 kJ + (0 kJ )] H f= -802.2 kJ Step #2: sum up all the H f. : Hrxn = Hf(products) - Hf(reactants) 16
Calculations Involving Specific Heat q = = C q C m T OR m T C = Specific Heat Capacity q = Heat lost or gained T = Temperature change Tf - Ti
Choose all that apply... C(s) + 2 S(g) CS2(l) H = 89.3 kJ Which of the following are true? A) This reaction is exothermic B) It could also be written C(s) + 2 S(g) + 89.3 kJ CS2(l) C) The products have higher energy than the reactants D) It would make the water in the calorimeter colder