Thermodynamics of Interaction-Free Ideal Gases in Canonical Ensemble
Explore the thermodynamics of interaction-free ideal gases within the canonical ensemble, focusing on classical Hamiltonian, Helmholtz free energy, quantum-mechanical considerations, and solutions for a monatomic ideal gas. Delve into the partition function, eigenenergies, and wave-number implications in the context of quantum mechanics and classical approximations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
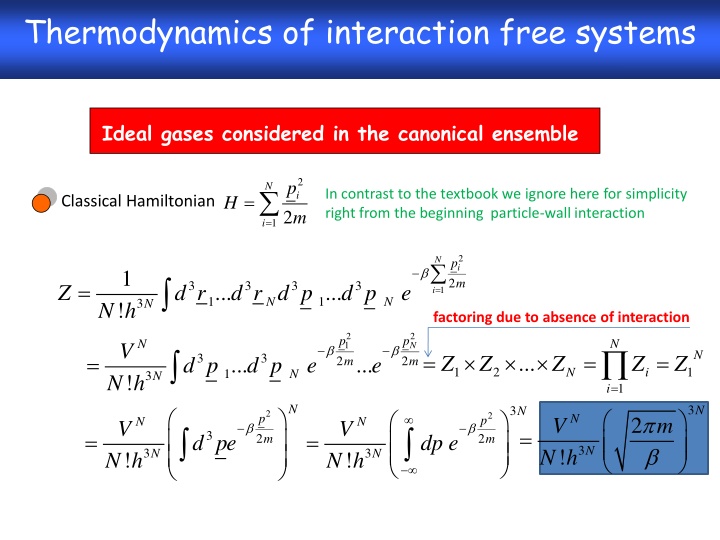
Thermodynamics of interaction free systems Ideal gases considered in the canonical ensemble 2 i m p N = In contrast to the textbook we ignore here for simplicity right from the beginning particle-wall interaction H Classical Hamiltonian 12 = i 2 i m N p 1 ! 12 = = 3 3 3 3 ... ... Z d r d r d p d p e i 1 1 N N 3 N N h factoring due to absence of interaction 2 1 m 2 N m p p N N V N = = = = ... 3 3 Z Z Z Z Z ... ... d p d p e e 2 2 1 2 1 N i 1 N 3 N ! N h = 1 i N 3 N 3 N = 2 2 p N 2 V m p N N V V = = 3 d pe dp e 2 2 m m 3 N ! N h 3 3 N N ! ! N h N h
3 N Helmholtz free energy & all thermodynamic information N 2 V m = Z With 3 N ! N h remember we showed ????? ??? ? = ?? = ??? ????+ ?? ??ln? = ???? + ??ln? ? ? ? ? = + ln TS k T U k T Z B B F = = ln U TS k T Z B
3 N 3 N 3 /2 N N 2 V T N h m N 2 V m = ln k T = = ln ln F k T Z k T B 3 N ! k B B 3 N ! N h B ( ) B k T N = + 3/2 ln VT const F T F V = = , S P = F V dF SdT PdV With V T 3/2 T = = P k TN = PV Nk T B 3/2 VT B T 1/2 3 VT 3 2 = F T V T ( ) ( ) = = + + = + + 3/2 3/2 ln ln S k N VT const k TN k N VT const k N B B B B 3/2 2 V = + With F U TS U F TS 3 2 ( ) ( ) = + = + + + + 3/2 3/2 ln ln U F TS k T N VT const k TN VT const k TN B B B 3 2 = U k T N B
We now consider a monatomic ideal gas of N atoms quantum mechanically p i with i i 2 i m 2 p 2 N N = = We go from the classical Hamilton function i H H 1 2 = m 12 = i i and the N particle Schroedinger equation 2 2 N ( ) ( ) = , ,..., , ,..., i r r r E r r r 1 2 1 2 N N 2 m = 1 i Again absence of interaction factoring in single particle problems We will later have a proper discussion of indistinguishable particles and symmetry of the N-particle wave functions Consider single particle Schroedinger equation ( ) ( ) 2 2 2 ( ( ( ) ) ) ( ( ( ) ) ) = = with boundary conditions 0, , , , 0 y z L y z m = r r n n x n n n = = ,0, , , 0 x z x L z n n y 3-dim particle in a box problem = = , ,0 x y , , x y L 0 n n z
remember 1-dim solutions 2 L nx L Eigenfunctions = ( ) x sin n 2 2 2 n Eigenenergies = = 1,2,3,... x n n x 2 2 mL x Quantum number x 2 2 ( ) = = = 1,2,3,... 1,2,3,... n n 2 2 2 n n n = + + 3-dim particle in a box problem n n n x x y z 2 2 mL , , x y z y 1,2,3,... n z Eigenenergies of N particle 3-dim. Schroedinger equation = = + + + ... E E , ,..., n n n n n n 1 2 1 2 N N = E Z e Partition function of canonical ensemble At this point we ignore symmetry constraints of wave-function/Pauli principle N 3 N ( ) + + + ... = = = Z e e e n n n n 1 2 N n , ,..., n n n n n 1 2 N
3 N 2 2 k m n n Introducing the wave-number n = = k Z e 2 L = 1 n With = = k k k + 1 n n L 3 3 N N 2 2 k m 2 2 k m L L n = Z e k dk e 2 2 0 k = 1 n 0 h mk T = Classical approximation appropriate for L th 2 B 2 2 k m With = mk T 2 dk e 2 B h 0 N 3 3 /2 3 N N 2 2 L h mk T h V = mk T = = N 2 B Z V B 3 2 th /2 N mk T h = = N ln ln F k T Z k T V B B B 2 ( ) 3/2 2 mk h 3 2 = B ln ln ln k TN V Nk T T Nk T B B B 3
( ) 3/2 2 mk h 3 2 = B ln ln ln F k TN V Nk T T Nk T B B B 3 Same free energy expression as obtained from classical partition function Same thermodynamics 3 2 = U k T N = PV Nk T B B 1 ( ) 1 3 N From we now understand origin of factor 3 in classical partition function = mk T 2 Z L N h B 3 N h Note: the origin of additional factor 1/N! to resolve Gibb s paradox requires an understanding of concept of indistinguishable quantum particles