Thermodynamics of Monatomic Ideal Gas
Dive into the quantum and classical perspectives of a monatomic ideal gas immersed in a heat bath, exploring concepts such as wavenumbers, energy levels, partition functions, free energy calculations, pressure, and entropy. Gain insights into the Gibbs paradox and key ideas related to continuum approximation and distinguishability of particles for accurate entropy calculations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
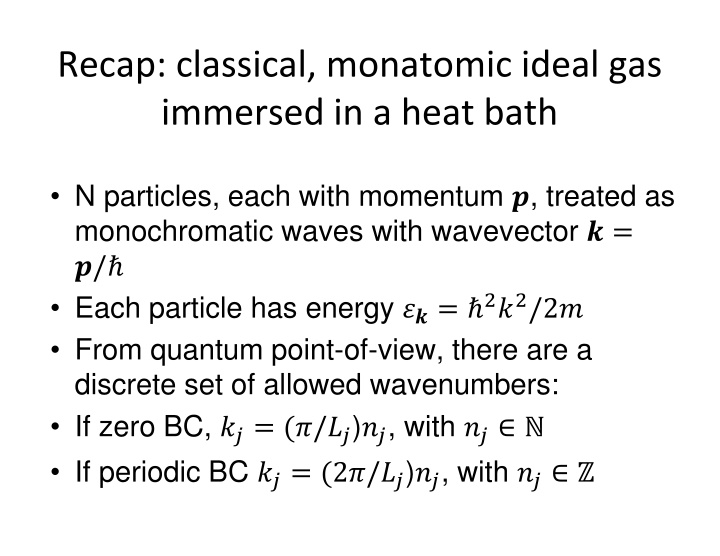
Recap: classical, monatomic ideal gas immersed in a heat bath N particles, each with momentum ?, treated as monochromatic waves with wavevector ? = ?/ Each particle has energy ??= 2?2/2? From quantum point-of-view, there are a discrete set of allowed wavenumbers: If zero BC, ??= (?/??)??, with ?? If periodic BC ??= (2?/??)??, with ??
Recipe for constructing thermodynamics 1. Identify microstates and corresponding energy levels 2. Construct partition function ? = ?? ??? 3. Calculate the free energy ? = ??? ln ? = ? ?? 4. Obtain pressure, entropy, etc. from F
Gibbs paradox: If gases are different (distinguishable), equivalent to two different gases expanding into vacuum + ? = ?(1)+ ?(2)= 2???ln(2?/?), so entropy is not additive!
SM6: key ideas Continuum approximation valid if spacing of energy levels very small compared to thermal energy Density of states = number of accessible microstates per energy (or wavenumber) Gibbs paradox => must be careful about distinguishability of particles to properly account for entropy of gases For classical ideal gas connected to heat bath, ? = ?1 Classical treatment valid for temperatures above degeneration temperature 3 ?/?!, with ?1= ?/??