Threshold for Platelets Study: Prospective Trial for Platelet Count in Critically Ill Patients
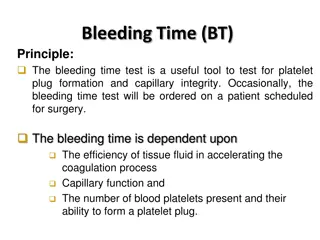
This study, conducted by Prof. Peter Watkinson at the University of Oxford, aims to determine the platelet count threshold necessitating platelet transfusion in critically ill patients before invasive procedures. It includes a list of participating hospitals, recruitment updates, and details of substantial amendments. The study discusses inclusion criteria modifications, such as specified low-bleeding risk invasive procedures. Follow the progress of this vital research in critical care.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
www.icnarc.org The Threshold for Platelets study: A prospective randomised trial to define the platelet count below which critically ill patients should receive a platelet transfusion prior to an invasive procedure All Site Meeting 11 January 2023 IRAS ID: 312405 REC Ref: 22/SC/0186 Funding: NIHR HTA (131822) NIHR CPMS ID: ISRCTN Registry: ISRCTN79371664 Sponsor: 53274 University of Oxford Chief Investigator: Prof Peter Watkinson All Site Meeting 1 V1.0 11.01.2023
Agenda Trial update Substantial amendment #1 Questions/site discussion www.icnarc.org All Site Meeting 2
Trial Update www.icnarc.org Date opened to recruitment Total Site name randomised 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 John Radcliffe Hospital Milton Keynes Hospital King s Mill Hospital (Sherwood) Poole Hospital University Hospital Coventry Tameside Russell s Hall Hospital Chesterfield Royal Hospital Croydon University Hospital Kettering General Hospital Blackpool Victoria Hospital Royal Liverpool Hospital Basingstoke Hospital Churchill Hospital Royal Cornwall Hospital Royal Hampshire County Whittington Hospital Great Western Hospital 27.09.2022 24.10.2022 28.09.2022 05.12.2022 05.12.2022 24.10.2022 05.12.2022 17.11.2022 19.12.2022 05.10.2022 07.11.2022 13.12.2022 14.11.2022 20.10.2022 17.10.2022 14.11.2022 06.12.2022 19.12.2022 5 4 3 3 2 2 1 1 1 1 1 1 0 0 0 0 0 0 Open sites: 18 Recruitment to date: 25 Recruitment in January so far: 5 25 All Site Meeting 3
Agenda Trial update Substantial amendment #1 Questions/site discussion www.icnarc.org All Site Meeting 4
Substantial Amendment #1 www.icnarc.org Inclusion criterion no.4 Previous wording: o Platelet transfusion being considered for a low bleeding risk invasive procedure New wording: o Planned to undergo a specified* low bleeding risk invasive procedure OR platelet transfusion being considered for an other procedure All Site Meeting 5
Substantial Amendment #1 Inclusion criterion no. 4 (cont.) www.icnarc.org *Specified low bleeding risk invasive procedures include the following: o Central venous vascular catheter insertion (including vascular access for renal replacement therapy) o Paracentesis/superficial abdominal fluid collection drainage o Pleural aspiration All Site Meeting 6
Substantial Amendment #1 Inclusion criterion no. 4 (cont.) www.icnarc.org Other procedures may be included if the clinician deems these to be a low bleeding risk invasive procedure and a platelet transfusion is being considered for the procedure. These include, but are not limited to, the following: o Arterial catheter insertion o Arterial or central venous catheter removal o Pleural drain o Interventional radiology o Bronchoscopy with or without lavage o Wound dressing changes o Surgical procedures where the clinical team agree risk of bleeding is low, e.g., re-look laparotomy, or wound closure All Site Meeting 7
Substantial Amendment #1 Exclusion criteria updates Addition of acute promyelocytic leukaemia (APML) Minor wording change Known advance decision refusing blood/blood component transfusions (e.g., Jehovah s Witnesses) www.icnarc.org Safety reporting process updates Updates to the way you report a SAE have been reflected in the protocol o No longer need to submit a paper SAE Report Form o Report SAE directly onto MACRO PI sign off is electronic This process has already been implemented on MACRO All Site Meeting 8
Substantial Amendment #1 What happens next? Implementation date: 12 January 2023 (tomorrow!) Sealed Envelope will deploy changes to eligibility criteria o Once deployed, we will send the implementation email to sites Documents to be implemented: o Protocol V2.0 o Randomisation Form V2.0 o Screening Log V2.0 o Screening Tool V2.0 (optional) o SOP003 Patient Screening V2.0 o ISF Checklist V2.0 www.icnarc.org P.S. New laminated staff posters will be posted out All Site Meeting 9
Questions/site discussion Any questions? www.icnarc.org T4P@icnarc.org 020 7269 9277 Icnarc.org/Our-Research/Studies/T4P All Site Meeting All Site Meeting 10