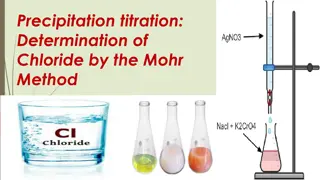
Titration Techniques and Calculations
Learn about titration, a crucial technique in determining unknown solution concentrations. Explore acid-base titrations, monitoring methods, calculation processes, and essential elements of titration. Discover how titration is used to find the concentration of unknown substances accurately.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration (called the analyte) until the reaction reaches neutralization. Titration is a technique to determine the concentration of an unknown solution. Acid-Base titrations are usually used to find the amount of a known acidic or basic substance through acid base reactions.
Monitoring titrations Titrations can be monitored Instrumentally with pH meters With litmus paper Using indicators that change color at the endpoint The horizontal lines show the range of pH in which phenolphthalein (blue) and methyl orange (red) changes color. The middle line represents the pKa, while the two outer lines represent the end or start of the color changes. The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added.
Titration Calculation The video described titration of a strong acid with a strong base H2SO4(aq) + 2NaOH (aq) 2H2O (l) + Na2SO4(aq) The molarity of the titrant was 0.1002 M NaOH and the amount of NaOH(aq) added was 9.57 mL so the number of moles of NaOH added was 0.1002 M x 0.00957 = 0.00959 moles Since each mole of NaOH neutralizes mole of H2SO4 the number of moles of acid that we started with is 0.00479 moles. To find the molarity of the H2SO4 we simply divide the number of moles H2SO4 by the volume of the analyte we started with
Elements of Titration The standard solution is the solution of known concentration. An accurately measured amount of standard solution is added during titration to the solution of unknown concentration until the equivalence or endpoint is reached. The equivalence point is when the reactants are done reacting. The solution of unknown concentration is otherwise known as the analyte. During titration the titrant is added to the analyte in order to achieve the equivalence point and determine the concentration of the analyte. The equivalence point is the ideal point for the completion of titration. In order to obtain accurate results the equivalence point must be attained precisely and accurately. The solution of known concentration, or titrant, must be added to the solution of unknown concentration, or analyte, very slowly in order to obtain a good result. At the equivalence point the correct amount of standard solution must be added to fully react with the unknown concentration. The end point of a titration indicates once the equivalence point has been reached. It is indicated by some form of indicator which varies depending on what type of titration being done. For example, if a color indicator is used, the solution will change color when the titration is at its end point.
Titration of a Weak Acid With a Strong Base For example the titration of acetic acid with NaOH C2H4O2(aq) + OH-(aq) C2H3O2-(aq) + H2O(l) in general we can represent such a titration as HA + OH- A- + H2O At the equivalence point all of the acid has been converted to A- and the A- will hydrolyze water A- + H2O = HA + OH- Thus at the equivalence point the pH will be greater than 7
Titration of a Polyprotic Acids Equivalence point. All the acid has been neutralized Midpoint at which [A-] and [HA] are equivalent pH=pKa Slow increase as presence of A- and HA acts as buffer Initial increase as acid is converted to A-
Calculating pH During a Titration Let us start by calculating the pH of a solution of 0.100 M acetic acid C2H4O2(aq) C2H3O2-(aq) + H+ (aq) [C2H3O2-] [H+] 1.00x 10-7 [C2H4O2] 0.100 0 Initial Change Final ? = 1.74 ? 10 5=[C2H3O2 ][H+] = [C2H4O2] pH = -log10[H+] =
Calculating pH During a Titration If we add 5.00 mL of 0.200 M NaOH to 50.00 mL of 0.100 M acetic acid then some of the acetic acid will be neutralized. This becomes a stoichiometry problem. We need to a. Write the balanced chemical reaction C2H4O2(aq) +OH- C2H3O2-(aq) + H2O(l) b. Calculate the number of moles of both reactants in the reacting solutions nAc= 0.0500 L x 0.100 M = nNaOH = 0.005 L x 0.200 M = c. Determine how much of each is left and how much of the [C2H4O2] is left (not converted to C2H3O2+)
Calculating pH During a Titration Since this is a stoichiometry problem, we need to follow the number of moles of each species, not the molarity, but we will use the same table. This can be confusing. Take care nAcetic Acid nhydorxide ion nAcetate ion Initial Change Final However to get the pH we will have to calculate the molarity, which means that we need the FINAL volume after mixing the NaOH solution and then the concentrations of C2H4O2(aq) and C2H3O2-(aq) Vf= Vacetic acid + VNaOH= 50.00 mL + 5.00 mL= 55.00 mL
Calculating pH During a Titration [C2H4O2]=?C2H4O2 [C2H3O2 ]=?C2H3O2 0.055 ? 0.055 ? With the concentrations of C2H4O2(aq) and C2H3O2-(aq) immediately after adding NaOH we now have to solve an acid equilibrium problem [C2H3O2-] [H+] [C2H4O2] Initial Change Final ? = 1.74 ? 10 5=[C2H3O2 ][H+] = [C2H4O2] pH = -log10[H+] =
Calculating pH at the Midpoint At the midpoint [C2H4O2]=[C2H3O2-] so [H+]mp = Ka ?? =[C2H3O2 ][H+] [C2H4O2] = [H+]mp and pHmp= pKa [C2H3O2-] [H+] [C2H4O2] Initial Change Final
Calculating pH at the Equivalence Point At the equivalence point an equivalent amount of the base titrant has been added to the acid analyte. The pH can be found from an equilibrium analysis assuming that [C2H3O2-]eq = [C2H4O2]o and that initially [H+] =[C2H4O2]=0. The reaction is the hydrolysis reaction C2H3O2-(aq) + H2O(l) = C2H4O2(aq) + OH- (aq) and [C2H3O2-] [C2H4O2] Initial Change Final [OH-] 0 0 [C2H4O2] ?? ??= ??= [C2H3O2 ][OH ][C2H4O2] = pH = 14 - pOH
Summary A similar analysis holds for the titration of a weak base by a strong acid. Calculation of pH at all points along the titration curve is a combination of stoichiometric and equilibrium problems
Titration of Polyprotic Acids Deprotonation of H2PO4- Deprotonation of H3PO4