Understand Halocarbons and Naming Conventions
Learn about halocarbons, their properties, and naming conventions involving halogens. Discover the positioning of halogens on the periodic table, functional groups, and how to name compounds with halogen substitutions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
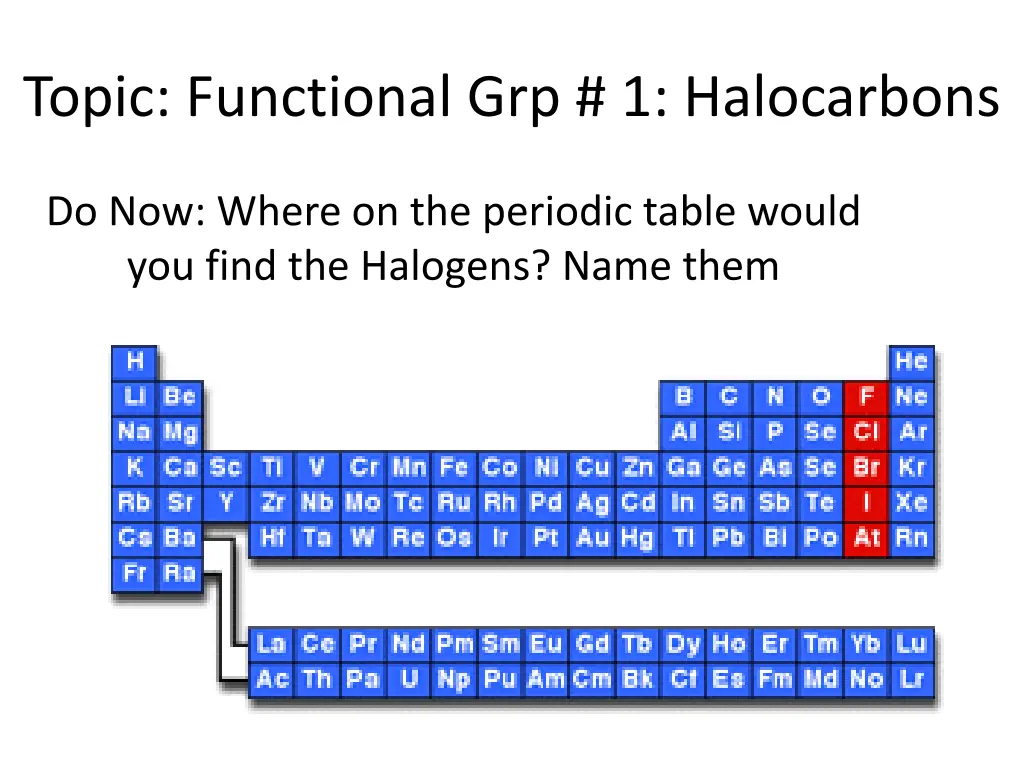
Topic: Functional Grp # 1: Halocarbons Do Now: Where on the periodic table would you find the Halogens? Name them
aka Alkyl Halides, Organic Halides One (or more) hydrogen atoms in alkane is replaced with halogen atom (F, Cl, Br, or I) propane
General Formula halocarbons: R-X R = hydrocarbon part of molecule X represents halogen (F, Cl, Br, or I)
Properties Boiling Point ( C) Adding a halide increases IMF Higher BP/MP CH3CH2CH2CH2CH3 pentane 36 CH3CH2CH2CH2CH2F 1-fluoropentane 63 The larger the halide the stronger the IMF I>Br>Cl>F CH3CH2CH2CH2CH2Cl 1-chloropentane 108 CH3CH2CH2CH2CH2Br 1-bromopentane 130 CH3CH2CH2CH2CH2I 1-iodopentane 155
Naming figure out backbone name prefixes specify substituent: fluoro, chloro, bromo, iodo use di, tri, tetra if more than one same thing tell location(s) of halogen(s) state # C attached to in backbone
C3H7F CH3Cl CH3CHFCH3 H H C Cl H 2-fluoropropane chloromethane
CH3CCl2CHClCH3 C4H7Cl3 Naming Halides 1,2,2-trichlorobutane
If more then one kind of Halogen, name them alphabetically 1st alphabetically gets lowest number 4 3 2 1 2-chloro-4-fluoro-3-iodobutane
Name: Br CH3 CH2 CH CH CH3 I 3-bromo 2-iodopentane F Cl H C C H F Cl 1,1-dichloro-2,2-difluoromethane


