Understanding Alcohols, Ethers, Amines, and Carboxylic Acids
Explore the properties of alcohols, ethers, amines, and carboxylic acids, including their molecular structures, functional groups, and hydrogen bonding capabilities. Learn how these chemical compounds differ and affect factors like boiling points and reactivity.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
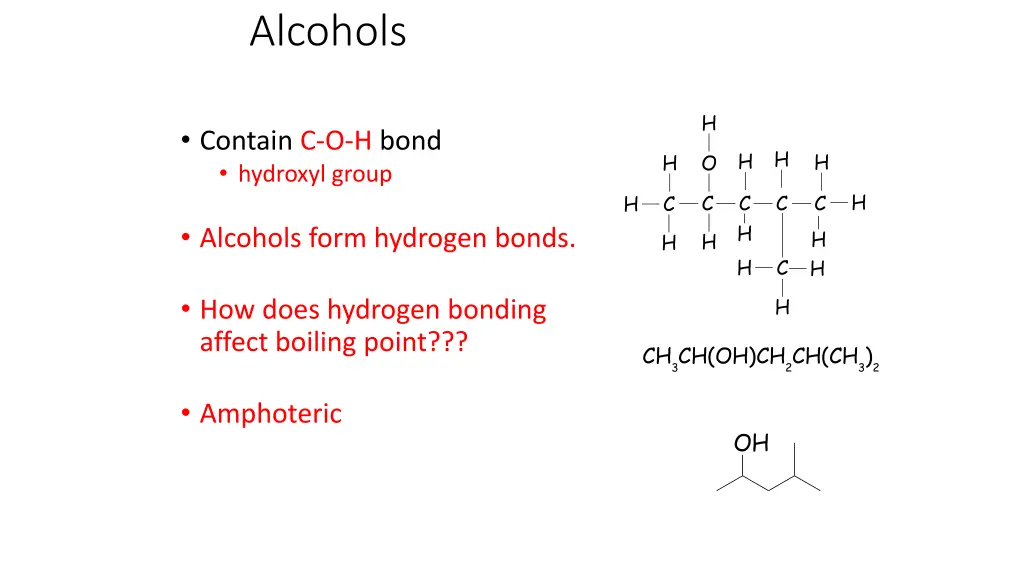
Alcohols CH3CH(OH)CH2CH(CH3)2 CH3CH(OH)CH2CH(CH3)2 H Contain C-O-H bond hydroxyl group H H H H O H H H C C H H C C H H C C H H H H O Alcohols form hydrogen bonds. H H H C C C H H C C H H H H How does hydrogen bonding affect boiling point??? H H C OH CH3CH(OH)CH2CH(CH3)2 H Amphoteric OH H H H H H O H C C H H C C H H C H H H C H OH
CH3CH(OH)CH2CH(CH3)2 CH3CH2OCH2CH3 CH3CH(OH)CH2CH(CH3)2 Ethers H O (CH3CH2)2O H H O H H Contain C-O-C bond C C H CH3CH2OCH2CH3 H H C H C O C C H C H H C H H (CH3CH2)2O tetrahedral e.d. geometry H H H H H H H H CH3CH(OH)CH2CH(CH3)2 CH3CH(OH)CH2CH(CH3)2 C C H H H H H H CH3CHO H O C C H O CH3CH2OCH2CH3 CH3CH2OCH2CH3 H H H HH OH (CH3CH2)2O (CH3CH2)2O H C C H O C O C H H H H H H H H H H H C O C C H C H H C O C C H C H OH O H H H H H H bent molecular geo. OH O OH O
Amines H H H H H H H H H H H H H C H C H C H H H H H H H H C C H C H C N H C C C H C N C H Contain C-N-R H C H C H C C C N C H H H C C C C H H C H H N H H C H H H H H H H H H H H H H H H H R H H H H CH3CH2CH2CH2CH2NHCH3 R and R can be H or C CH3CH2CH2CH2CH2NHCH3 CH3CH2CH2CH2CH2NHCH3 CH3CH2CH2CH2CH2NHCH3 H H H H Primary and secondary amines form hydrogen bonds. N N N N Common organic bases lone pair of e-on N CH3NH2 CH3NHCH3 (CH3)3N 1 primary secondary o 2 o 3 o tertiary
H O H O Aldehydes H H C C C H C H O H O H H C - H Contain (-CHO) Carbonyl (C=O) always on the 1st or last carbon in a chain H C C H H CH3CHO CH3CHO O O CH3CHO H H O trigonal planar geometry sp2 hybrid orbitals H O O H H C C H H C H C H H H CH3CHO O H
CH3C(O)CH3 H O H O CH3C(O)CH3 H H C C C HH H C Ketones H C C H H H O H O O H H H C H Contain Carbonyl attached to middle of chain C C C-C-C HH H C H CH3CHO C C H H H H O H H CH3C(O)CH3 H CH3C(O)CH3 C CH3CHO H C H O H O H H H O H HH H O Trigonal planar e.d. geo. H O H C H C C H C C H C H H H C HH C H C H C C H C H H C C H H H H H sp2 hybridized C H H CH3CHO CH3CHO O H H O H H H H C H H C H C C H H
H H H H H H H H H H H H H C C C C C C C N C C H H C C C N H H H H H H H H H H H H H H H CH3CH2CH2CH2CH2NHCH3 CH3CH2CH2CH2CH2NHCH3 H H N N H O H Carboxylic Acids Contain carboxyl group C H C H C O H H CH3NH2 CH3NHCH3 (CH3)3N o 2 CH3NH2 CH3NHCH3 (CH3)3N 1 1 H H O H H o H C H O O C H o 3 H H o 2 o 3 C H o C O H C H C H C H C CH3CH2CO2H O H C H O H H -CO2H -COOH -CO2H -COOH Form hydrogen bonds OH CH3CH2CO2H CH3CH2CO2H CH3CH2CO2H O trigonal planar OH OH H H O OH O H C H sp2 hybridized carbon C C H O
H O H C H C H C O H H CH3CH2CO2H H H H OH C H C C H Esters O H H O H H H H O H H O H H H H C H C H O H O H C H H C H C C H O H C H C H C H H C C H H C H C C H O H C H Contain C H H H C H H H C H C H O C H -CO2R where R = alkyl group CH3CH2CO2CH2CH3 CH3CH2CO2CH2CH3 CH3CH2CO2CH2CH3 CH3CH2CO2CH2CH3 O O O O H H H H O O H H H H H O H H O H H trigonal planar H C H O H C C H O H H C H O H C C H C O C HH HH C C H H C H HH HH C C C H C H C H H C H C C C H O H O O sp2 hybridized O -CO2R where R = alkyl group -CO2R where R = alkyl group -CO2R where R = alkyl group -CO2R where R = alkyl group
Amides H O H H H H O C H C H H H H O H H C H H Contain where R and R = H or C C H N H H C H H C H C H C N H C H H C H C H H C H C H C N H H C H H CH3CH2CONHCH2CH3 CH3CH2CONHCH2CH3 O CH3CH2CONHCH2CH3 C=O is trigonal planar & sp2 hybridized H O H H H O H H N O H H C O O C H H H H H C HH HH O C C C C H H H H C H H H C C H H O O H C O C H C HH C C H O -CO2R where R = alkyl group -CO2R where R = alkyl group -CO2R where R = alkyl group
H O H H H C H C H C N H C H H C H H H O H H H C H C H C N H C H H C H H CH3CH2CONHCH2CH3 H N CH3CH2CONHCH2CH3 H H O H H H H H O H H N C O C C H H C H C HH C C H C C N H H C H H Nitriles H H H H O H O H H C O C Contain H H C HH C C H CH3CN H O O O CH3CH2CONHCH2CH3 H O Linear CH3CNH2 CH3CNHCH3 CH3CN(CH3)2 N O O o 2 H o 3 O o O sp hybridized C 1 H O C C HH H H CH3CNH2 CH3CNHCH3 CH3CN(CH3)2 H C C H H H C H C N C H N o 2 H o 3 H o CH3CN H 1 H O N C H C H C N H C H CH3CN H O O O CH3CNH2 CH3CNHCH3 CH3CN(CH3)2 o 2 H o 3 H o 1 N C H C H C N H C H CH3CN H
Functional Groups Example: Identify the functional groups present in the following compounds. OH I I NH2 CH2CHCOOH HO O O I I thyroxine testosterone
