Understanding Allyl Chemistry: NBS Substitution Mechanism Explained
Explore the NBS substitution mechanism in allyl chemistry, focusing on radical and carbocation-based reactions, SN2 mechanism, factors governing product formation, and common electronic charge transfer behaviors. Discover the influence of steric hindrance and resonance in allylic site reactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
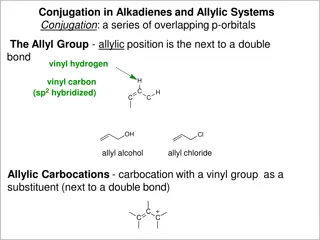
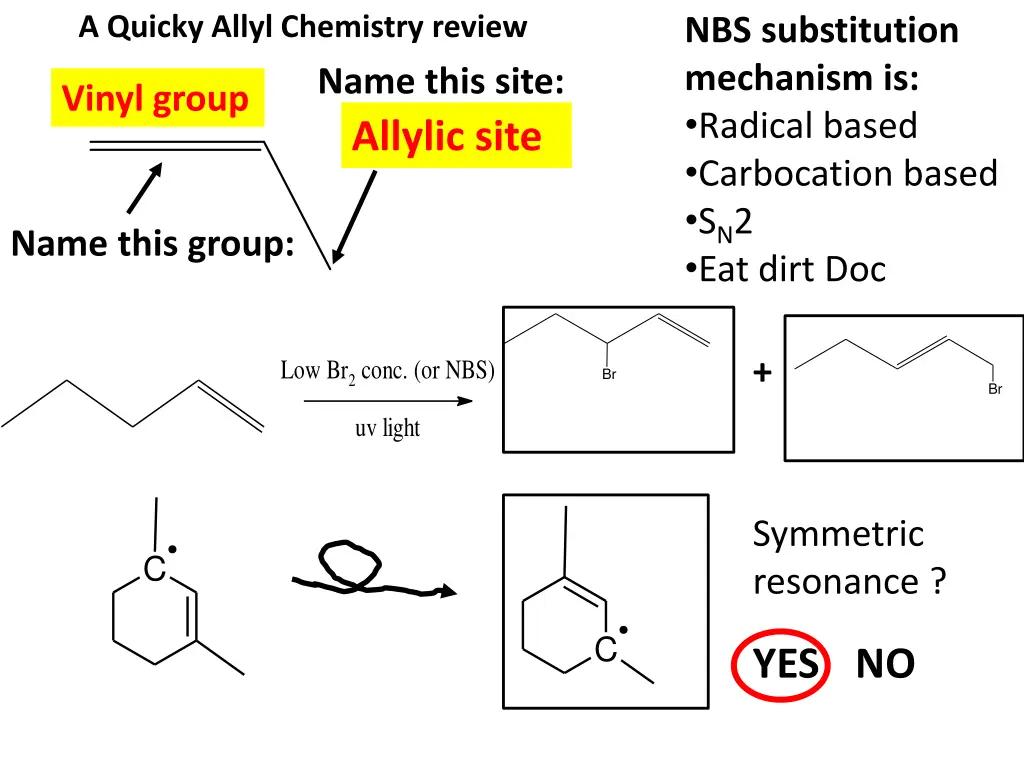
A Quicky Allyl Chemistry review NBS substitution mechanism is: Radical based Carbocation based SN2 Eat dirt Doc Name this site: Allylic site Vinyl group Name this group: Low Br2 conc. (or NBS) + Br Br uv light Symmetric resonance ? C C YES NO
A Quicky Allyl Chemistry review (continued) Which is more likely to form , A or B ? A B NBS + light Br Br What factor governs the choice of B as more likely above? Minimizing steric crowding (hindrance) What is the most likely product of: NBS Br light What factor governs the choice above? Radical intermediate forms symmetric resonance
The hydrolysis reaction shown is: Radical based Carbocation based SN1 Eat dirt Doc A Quicky Allyl Chemistry review (continued) NaHCO3 + Cl H2 O basic What is(are) the carbocation(s) in the above reaction ? A C+ B + CH2 Which is kinetically favored and why? A, since + charge is on 3o site Why is B thermodynamically favored ? More substituted around double bond (Zaitsev s rule)
A Quicky Allyl Chemistry review (continued) Both the allylic carbocation and radical mechanism share what common electronic charge transfer behavior ? Both feature electron shift towards radical or carbocation site to produce alternative intermediates C+ CH CH + CH2 radical allylic shit Carbocation allylic shift What governs the formation of the most stable carbocation above ? Carbocation degree (higher is better) What two factors govern formation of the most stable radicals above ? 1) Seeks symmetric resonance 2) Minimizes steric hindrance
https://mojocunanan.files.wordpress.com/2013/11/fuck-ya-happy-cat-meme-generator-did-you-say-vacation-heck-yeah-b10a7a.jpghttps://mojocunanan.files.wordpress.com/2013/11/fuck-ya-happy-cat-meme-generator-did-you-say-vacation-heck-yeah-b10a7a.jpg http://gladstonehotel.com/wp-content/uploads/2013/01/cat-vacation.jpg See you in 10 f***ing days