Understanding Atomic Structure and Quantum Mechanics
Explore the atomic structure of a Calcium atom, learn about Bohr models, orbitals, and energy levels. Discover the basics of Quantum Mechanics with educational resources and activities.
Uploaded on | 1 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Chemistry Jan 8, 2020 P3 Challenge- Consider a Calcium atom with 22 neutrons. Determine A) Draw the symbol complete with atomic number, mass number, and charge (if there is one.) B) Draw a Bohr model for the atom C) State the highest energy level and the number of valence elcetrons. Objective Atomic Structure/Quantum Mechanics Get out Bohr Model WS for HMK Check
Chemistry Jan 8, 2020 Objective Atomic Structure / Quantum Mechanics Agenda HMK review QM overview and video Organization of orbitals via Activity Assignment: Atomic Structure Exploration Activity
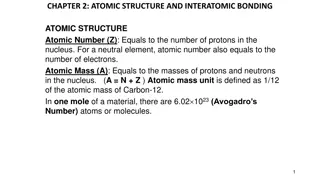
Quantum Mechanics Same as Bohr model except for how the energy levels are described. Electrons located in orbitals. Mathematical description is a wave function Schr dinger Explains the wave property of matter. Strategy to understand QM: Learn what the orbitals look like and how they re organized on H atom with activity (today) Populate the orbitals with electrons to describe other atoms using the periodic table (next time)
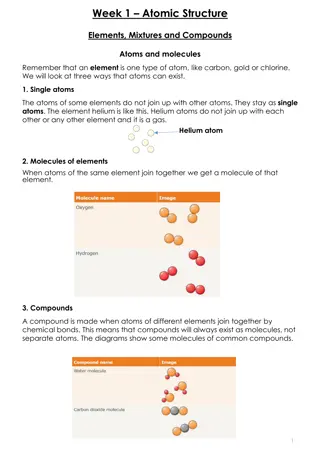
Shapes of Orbitals Using different shapes is one way QM creates its quantized energy levels. The more complicated the shape, the higher the energy level. Four different basic orbital shapes: s p d f Basic shapes double the number of lobes for each level: s, sphere p, dumbbell d, puffy 4 leaf clover f, complex
S, P, D, and F Orbitals Image result for d orbitals
Overview Video Orbitals, the Basics: Atomic Orbital Tutorial probability, shapes, energy; Crash Chemistry Academy https://www.youtube.com/watch?v=Ewf7RlVNBSA
Atomic Structure Exploration Activity Obtain a copy of the subshell sheets and an envelope, one set per lab table, a box of colored pencils and a pair of scissors. Complete an activity sheet for each team member. Due by end of period Submit an envelop of your atomic structure pieces labeled with all team member names Work in groups of 3-4 on the Atomic Structure Exploration Activity.
Exit Slip - Homework Exit Slip: What s Due? (Pending assignments to complete.) Atomic Structure Exploration Activity What s Next? (How to prepare for the next day) Read Holt p84 - 88