Understanding Atomic Structure and Radioactivity in Chemistry
Explore the world of atomic symbols, isotopes, mass numbers, neutrons, protons, and the concept of radioactivity in this informative content. Learn about nuclear reactions, transmutation reactions, and emissions from radioisotopes alongside intriguing visuals.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
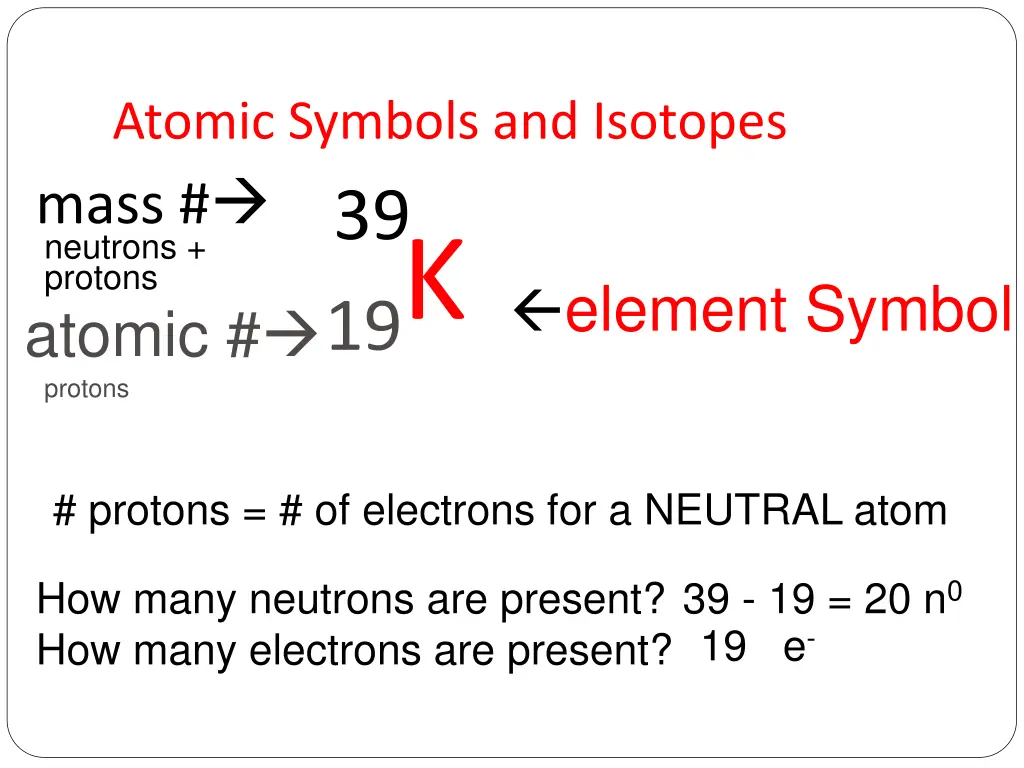
Atomic Symbols and Isotopes mass # neutrons + protons K 19 atomic # 39 element Symbol protons # protons = # of electrons for a NEUTRAL atom How many neutrons are present? How many electrons are present? 39 - 19 = 20 n0 19 e-
Complete the Table Below Atomic Number Mass Number Name Symbol Protons Neutrons 14 6C Carbon-14 6 8 6 14 41 19K Potassium-41 19 22 19 41 206 82Pb Lead-206 82 124 82 206
Radioactivity What is radioactivity? Radioactivity occurs when an unstable nucleus spontaneously emits fragments of the nucleus and/or energy. Unstable Nucleus = Radioactive Why are only some isotopes radioactive? The ratio of protons to neutrons in the nucleus determines whether or not a nucleus is radioactive.
Depicting Nuclear Reaction For nuclear reactions, the sum of the mass numbers (top numbers) and the sum of the atomic numbers (bottom number) must be the same on both sides. Mass Numbers: 9 + 4 = 12 + 1 13 = 13 Atomic Numbers: 4 + 2 = 6 + 0 6 = 6
Finding the Missing Nucleus He Th + 234 90 4 2 ? Mass Numbers 234 = 4 + ? ? = 230 230 88 Ra Atomic Numbers 90 = 2 + ? ? = 88
Finding the Missing Nucleus 1 6 + 14 0 A Z C e X Mass Numbers 14 = 0 + A A = 14 14 7 N Atomic Numbers 6 = -1 + Z Z = 7
Examples of Transmutation Reactions He + + 14 6 + 0 1 14 7 234 4 2 3 230 88 + C e N Th 90 He Ra 1 1 9 4 8 5 4 2 H Be B These are all transmutation reactions because the elements on the left side are changed to produce a different element on the right side.
Emissions from Radioisotopes Type of Particle Emitted Why is it Emitted? Description Symbol Mass Charge A Helium Nucleus A Fast Moving Electron Electromagnetic Radiation Nucleus is too large. Too many neutrons Too much energy 2 Alpha 4 amu +2 4 2 4 He or 1 amu Beta -1 0 1 0 1 e or 1840 0 0 Gamma 0 amu 0 A radioisotope is a nucleus that is radioactive or unstable.
Writing Nuclear Decay Equations Write the nuclear decay equation for the alpha decay of uranium-238. Start your decay equation by writing the symbol for this nucleus and then follow it with an arrow. of decay. Write the symbol for the type + 238 92 4 2 234 90 U He Th Mass Number: 238 = 4 + ? ? = 234 Atomic Number: 92 = 2 + ? ? = 90
Writing Nuclear Decay Equations Write the nuclear decay equation for the beta decay of iodine-131. Start your decay equation by writing the symbol for this nucleus and then follow it with an arrow. of decay. Write the symbol for the type + 131 53 0 1 131 54 I e Xe Mass Number: 131 = 0 + ? ? = 131 Atomic Number: 53 = -1 + ? ? = 54
Writing Nuclear Decay Equations Write the nuclear decay equation the emission of a gamma ray from carbon-14. Start your decay equation by writing the symbol for this nucleus and then follow it with an arrow. Write the symbol for the type of decay. + 14 6 0 0 14 6 C C Mass Number: 14 = 0 + ? ? = 14 Atomic Number: 6 = 0 + ? ? = 6
Examples of Transmutation Reactions He + + 14 6 + 0 1 14 7 234 4 2 3 230 88 + C e N Th 90 He Ra 1 1 9 4 8 5 4 2 H Be B These are all transmutation reactions because the elements on the left side are changed to produce a different element on the right side.
Decay Series for Uranium-238 This diagram shows the steps that an isotope of uranium takes to reach a stable isotope, lead-206.
A Closer Look at the Decay Series Write a nuclear decay equation for what we occur to uranium-234 according to this decay series. + 234 92 230 90 4 2 U Th He
A Closer Look at the Decay Series Write a nuclear decay equation for what we occur to lead-210 according to this decay series. + 210 82 210 83 0 1 Pb Bi e
Writing Nuclear Decay Equations Write the nuclear decay equation for the alpha decay of uranium-238. Start your decay equation by writing the symbol for this nucleus and then follow it with an arrow. of decay. Write the symbol for the type + 238 92 4 2 234 90 U He Th Mass Number: 238 = 4 + ? ? = 234 Atomic Number: 92 = 2 + ? ? = 90
Writing Nuclear Decay Equations Write the nuclear decay equation for the beta decay of iodine-131. Start your decay equation by writing the symbol for this nucleus and then follow it with an arrow. of decay. Write the symbol for the type + 131 53 0 1 131 54 I e Xe Mass Number: 131 = 0 + ? ? = 131 Atomic Number: 53 = -1 + ? ? = 54
Writing Nuclear Decay Equations Write the nuclear decay equation the emission of a gamma ray from carbon-14. Start your decay equation by writing the symbol for this nucleus and then follow it with an arrow. Write the symbol for the type of decay. + 14 6 0 0 14 6 C C Mass Number: 14 = 0 + ? ? = 14 Atomic Number: 6 = 0 + ? ? = 6
Protecting Ourselves from Radioactive Particles
Positive Uses of Nuclear Chemistry south-texas-nuclear-power-plant http://www.swedishamerican.org/images/heart_hospital/services/nuclear_medicine.jpg Nuclear Power Nuclear Medicine fruit Kills Bacteria on Food