Understanding Average Atomic Mass
Learn how to calculate the average atomic mass of elements through weighted averages of isotopes. Explore examples and practice problems to enhance your understanding of this fundamental concept in chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Average Atomic Mass http://tinyurl.com/md228mj Due Monday Oct 14, 2013
Objective Calculate the average atomic mass for an element
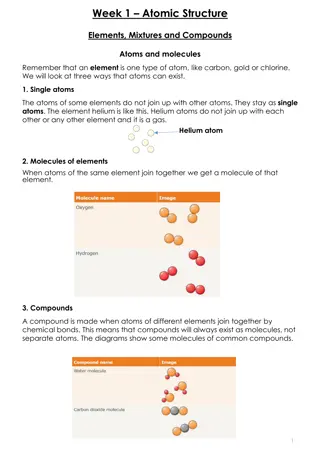
Average Atomic Mass The weighted average mass of all the isotopes found in nature for that element.
Average Atomic Mass Measured in atomic mass units (amu)
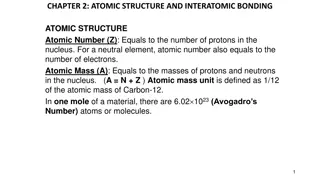
Mass Spectrometer Instrument that determines the percentages and individual masses of each isotope
Calculation The average atomic mass is calculated by the percent of each isotope s mass [(mass of isotope) (%abundance)] + [(mass of isotope) (%abundance)] + [(mass of isotope) (%abundance)]
Practice Problem 1 Boron has two isotopes: Boron-10 and Boron-11. In nature 19% of Boron is Boron- 10, and 81% is Boron-11.
Practice Problem 2 A new element, Tyserium (Ty), has recently been discovered on Mars and consists of two isotopes. One isotope has a mass of 331 amu and is 35.0 % abundant. The other isotope is 339 amu and is 65.0 % abundant. What is the mass of Ty as it appears on the periodic table?
Logic Problem #1 Copper has an average atomic mass of 63.546 amu and only exists as Copper- 63 and Copper-65. What is the isotopic mass of the most common isotope of Copper?
Logic Problem #2 The relative atomic mass of chlorine is 35.5 amu. What does this tell you about the relative abundance of the two naturally occurring isotopes of chlorine, Chlorine-35 and Chlorine-37.
Check Yourself! Make sure your calculated average atomic mass is within the range of the given mass numbers X-230, X-235, X-240 Rationalize your results 90% of X-230, 5% of X-235, 5% of X-240 Check your answers on the periodic table
Questions? If you have any questions, jot them down!