Understanding Carboxylic Acids and Their Derivatives
Explore the structures, nomenclature, common names, formulas, and properties of carboxylic acids and their derivatives. Learn about the classification of aliphatic and aromatic acids, resonance forms, IUPAC naming conventions, and more in this comprehensive guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
CARBOXYLIC ACIDS AND THEIR DERIVATIVES 1 340 Chem 1st 1439
Outline 2 Structure Nomenclature Carboxylic Acids derivatives Physical Properties Acidity Preparation of Carboxylic Acids Reaction 340 Chem 1st 1439
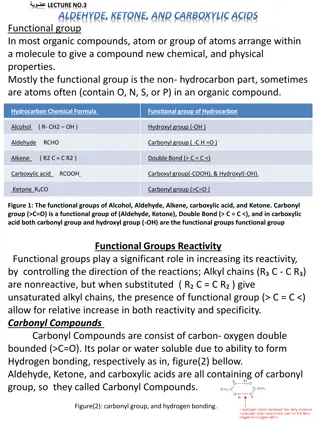
Structure 3 The combination of a carbonyl group and a hydroxyl on the same carbon atom is called a carboxyl group (-CO2H or COOH). Carboxylic acids are classified according to the substituent bonded to the carboxyl group. An aliphatic acid has an alkyl group bonded to the carboxyl group, and an aromatic acid has an aryl group. The simplest acid is formic acid, with a hydrogen atom bonded to the carboxyl group. Fatty acids are long-chain aliphatic acids derived from the hydrolysis of fats and oils. 340 Chem 1st 1439
4 The following resonance forms can be written for a carboxyl group. 340 Chem 1st 1439
Nomenclature 5 IUPAC name The root name is based on the longest continuous chain of carbon atoms bearing the carboxyl group. The -e is replaced by -oic acid. The chain is numbered starting with the carboxyl carbon atom. Cycloalkanes with carboxyl substituents are named as cycloalkanecarboxylic acids. 340 Chem 1st 1439
common name 6 C-C-C-C-C-COOH In common names, the positions of substituents are named using Greek letters. Notice that the lettering begins with the carbon atom next to the carboxyl carbon, the carbon. CH3 commmone: , - Dimethyl butyric acid IUPAC: 2,3-Dimethyl butanoic acid C H3 C H3 COOH B r C H3C H2C H2C H C O O H -Bromo valeric acid 340 Chem 1st 1439
No. C Formula IUPAC Common 7 1 2 3 4 5 HCOOH CH3COOH CH3CH2COOH CH3(CH2)2COOH CH3(CH2)3COOH Methanoic acid Ethanoic acid Prpanoic acid Butanoic acid Pentanoic acid Formic acid Acetic acid Prpionic acid Butyric acid valeric acid 340 Chem 1st 1439
COOH COOH COOH COOH 8 COOH COOH 1,4-Benzenedicarboxylic acid 1,2-Benzenedicarboxylic acid 1,3-Benzenedicarboxylic acid (Terephthalic acid) (Phthalic acid) (Isophthalic acid) trans-3-Phenylpropenoic acid (Cinnamic acid) COOH 3- Cyclohexene carboxylic acid 340 Chem 1st 1439
9 340 Chem 1st 1439
Physical Properties 10 Solubility Carboxylic acids interact with water molecules by hydrogen bonding through both the carbonyl and hydroxyl groups. Because of greater hydrogen bonding interactions, carboxylic acids are more soluble in water than are alcohols, ethers, aldehydes, and ketones of comparable molecular weight. A carboxylic acid consists of two regions of distinctly different polarity: a polar hydrophilic carboxyl group and, a nonpolar hydrophobic hydrocarbon chain. The hydrophilic carboxyl group increases water solubility; the hydrophobic hydrocarbon chain decreases water solubility. As the size of the hydrocarbon chain increases relative to the size of the hydrophilic group, water solubility decreases. 340 Chem 1st 1439
11 Boiling Point Carboxylic acids have significantly higher boiling points than other types of organic compounds of comparable molecular weight, such as alcohols, aldehydes, and ketones. The hydroxyl group of one carboxylic acid molecule acts as a proton donor toward the carbonyl oxygen of a second. In a reciprocal fashion, the hydroxyl proton of the second carboxyl function interacts with the carbonyl oxygen of the first. The result is that the two carboxylic acid molecules are held together by two hydrogen bonds. So efficient is this hydrogen bonding that some carboxylic acids exist as hydrogen-bonded dimers even in the gas phase. In the pure liquid a mixture of hydrogen-bonded dimers and higher aggregates is present. 340 Chem 1st 1439
Acidity 12 Carboxylic acids are the most acidic class of compounds, they are much stronger acids than water and alcohols. Carboxylic acids are weak acids. Dissociation of either an acid or an alcohol involves breaking an O- H bond, but dissociation of a carboxylic acid gives a carboxylate ion with the negative charge spread out equally over two oxygen atoms, compared with just one oxygen in an alkoxide ion .This charge delocalization makes the carboxylate ion more stable than the alkoxide ion; therefore, dissociation of a carboxylic acid to a carboxylate ion is less endothermic than dissociation of an alcohol to an alkoxide ion. COOH COOH OH OH increase acidity 340 Chem 1st 1439
Electron-withdrawing groups enhance acidity because they help to stabilize the negative charge of the conjugate base (the carboxylate ion). The amount of stabilization depends on: 1. the number of electron-withdrawing groups; 2. the strength of the electron-withdrawing groups; and 3. the distance of the electron-withdrawing groups from the COOH group. 13 340 Chem 1st 1439
14 Electron donating substituents decrease the acidity HCOOH > CH3COOH > CH3CH2COOH > CH3CH2CH2COOH Whereas Electron withdrawing substituents near the carboxyl group increase the acidity Cl3CCOOH > Cl2CHCOOH > ClCH2COOH > CH3COOH COOH COOH COOH COOH HOOC > > > > O2N NO2 NO2 CH3 H3 C 340 Chem 1st 1439
15 340 Chem 1st 1439
Carboxylic acid derivatives O 16 R O(K or Na) Salts all carboxylic acid derivatives consist of an acyl group (R-C=O) attached to an electronegative atom 340 Chem 1st 1439
Nomenclature of Acyl Chlorides 17 Replace the -ic acid ending in the name of the parent acid by yl chloride. O O O H3C Cl Cl CH3CH2 C Cl IUPAC: Ethanoyl chloride Benzoylchloride Common : Acetyl chloride Propanoyl chloride 340 Chem 1st 1439
Nomenclature of Esters 18 The names of esters are derived from the names of the alcohol (with the ending -yl) and the acid (with the ending -ate or -oate). O CH3CH2 C OCH3 O HO Methyl propanoate Isopropyl-4-hydroxy-5- (IUPAC) Ethyl ethanoate Methyl benzoate (common) Ethyl acetate 340 Chem 1st 1439 O methyl-5- hexenoate
Nomenclature of Amides 19 Amides that have no substituent on nitrogen are named by dropping -ic acid from the common name of the acid (or -oic acid from the substitutive name) and then adding -amide. Alkyl groups on the nitrogen atom of amides are named as substituents, and the named substituent is prefaced by N- or N,N-. (IUPAC) Ethanamide Benzamide (common) Acetamide O CH3CH2 N-Methylpropanamide N-Methylpropionamide C NHCH3 N,N-Dimethylmethanamide N-Ethyl-N-methylbenzamide N,N-Dimethylformamide 340 Chem 1st 1439
Nomenclature of Anhydrides 20 Most anhydrides are named by dropping the word acid from the name of the carboxylic acid and then adding the word anhydride (IUPAC) Ethanoic anhydride (Common) Acetic anhydride Benzoic anhydride Butandioic anhydride Succinic anhydride O O OH heat O OH O O 340 Chem 1st 1439
Preparation of Carboxylic Acids By oxidation of aldehydes and primary alcohols. 21 K M n O4 / H + P C C C H3C O O H C H3C H2O H C H3C H O COOH CH2OH CHO heat KMnO4 / H+ or K2Cr2O7 /H+ Cu / heat 340 Chem 1st 1439
By oxidation of alkylbenzenes 22 NO2 NO2 CH3 KMnO4 / H+ or K2Cr2O7 /H+ COOH CH2CH2CH3 COOH KMnO4 / H+ or K2Cr2O7 /H+ 340 Chem 1st 1439
By hydrolysis of cyanohydrins and other nitriles 23 H+ R-COOH R-X + NaCN RCN +H2O heat (K,Na,..) OH RCOO (K<Na..) 1)Li AL H4, ether 2)H3O or H2 / Pt RCH2NH2 amine 340 Chem 1st 1439
carbonation of Grignard reagents. 24 Grignard reagents react with carbon dioxide to yield magnesium carboxylates. Acidification produces carboxylic acids H3O CO2 2) 1) RCOOH R-COOMgX R-Mg X CH3-COOMgBr2) H3OCH3COOH 1)CO2 CH3-Mg Br + Mg(OH)Br 340 Chem 1st 1439
The haloform reaction 25 (converts methyl ketones to acids and iodoform O R + 3I2 + 4 NaOH CHI3 + 3NaI +RCOONa + 3H2O CH3 340 Chem 1st 1439
Kolbe-Schmitt Carboxylation using in synthesis of salicylic acid 26 340 Chem 1st 1439
Reaction of Carboxylic Acids Reaction with Bases 27 KOH or NaOH RCOO-K+ or Na++ H2O RCOO-Na++ NaHCO3 + RCOOH CO2 H2O RCOO-NH4 + NH3 COOH COO Na NaHCO3 + CO2 + H2O Sodium benzoate OH NaHCO3 N. R 340 Chem 1st 1439
Nucleophilic Substitution of Hydroxyl Group O 28 O + + Nu L R L R Nu R'OH / H+ RCOOR' Ester NH3 RCONH2 Amide SOCl2 RCOOH Acid Chloride RCOCl 1) PCl5 2) R''COO-Na+ RCOOCOR" Anhydride 340 Chem 1st 1439
Formation of Easter Fischer Esterification 29 R'-OH / H+ heat R-COO-R' + H2O RCOOH OH COOC2H5 OH CH3CH2OH H+ / heat COOH Formation of amide NH3 or R-NH2 ,heat NH3 , RCOOH CH3COOH R-CONH2 CH3-CONH2 heat 340 Chem 1st 1439
Formation of acid anhydride: -H2O 30 O O H+ RCOO-H + HO-OCR' + H2O R O R' O O H2SO4 R-C-O-C-R O 2 RCOOH O H2SO4 2 CH3CH2COOH Formation of acid chloride: CH3CH2-C-O-C-CH2CH3 340 Chem 1st 1439
31 340 Chem 1st 1439
Preparation of acid derivatives from acid chloride 32 O C H3O H O C H3 O O C H3N H2 C l N H -C H3 O O O O O N a 340 Chem 1st 1439
Reaction of Derivatives of carboxylic acid 33 On hydrolysis (reaction with H2O) all carboxylic acid derivatves convert to carboxylic acid. O H3O+ R O O R H3O+ O R O O H R O O H3O+ R NH2 or eq NaOH/H3O+ O H3O+ R Cl 340 Chem 1st 1439
Overview Reaction 34 340 Chem 1st 1439
AMIDES 35 340 Chem 1st 1439
Esters 36 340 Chem 1st 1439
Fischer Mechanism (1) 37 Protonation of carbonyl and attack of alcohol, a weak nucleophile. 340 Chem 1st 1439
ANHYDRIDES 38 340 Chem 1st 1439
Reactions of acid derivatives 39 a) Acid chlorides: O H2O/ H+ R-C-OH + HCl O R'OH R-C-OR' + HCl O R-C-Cl O NH3 R-C-NH2 + HCl O R'NH2 R-C-NHR' + HCl O R' R' R'2NH R-C-N + HCl Reduction: O 1) Li Al H4 / ether 2) H3O R-CH2OH R 340 Chem 1st 1439 Cl
B] Acid anhydride: 40 340 Chem 1st 1439
C] Esters: H+ 41 RCOOH + R'OH RCOOR' + H2O H+ RCOOR'' + R'OH RCOOR' + R''OH H+ RCONH2+ R'OH RCOOR' + NH3 Reduction: 1)Li Al H4 / ether 2) H3O RCH2OH+ R'OH RCOOR' 1)Li Al H4 / ether 2) H3O CH3-CH2COOCH3 CH3CH2CH2OH+ CH3OH OH 1) 2 R'' Mg X 2 ) H3O / H+ R-C-R'' R'' R-COO-R' + R' OH 340 Chem 1st 1439
d] Amide: 42 1-Reduction: O O H+ + H2O R-C-OH + NH3 R-C-NH2 O O / heat NaOH R-C-ONa + NH3 R-C-NH2 O 1) Li Al H4 / Ether 2) H3O R-CH2NH2 R-C-NH2 O 1) Li Al H4 / Ether 2) H3O R-CH2NHR' R-C-NHR' O R' R' 1) Li Al H4 / Ether 2) H3O R-CH2NR'2 R-C-N 340 Chem 1st 1439
2-Dehydration: 43 O P2O5 - H2O R-C-NH2 R-CN nitrile 3-Hoffman degradation: O Br2/ N aO H or N aO Br R-CH2N H2 RCH2-C-N H2 O B r2/ N aO H C H3-C -N H2 C H3N H2 340 Chem 1st 1439