Understanding Chemical Reactions and Balancing Equations
Learn about chemical reactions, Dalton's atomic theory, and the importance of balancing chemical equations. Discover how to represent reactions as equations, understand coefficients, and why balancing is necessary to maintain the law of conservation of mass.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
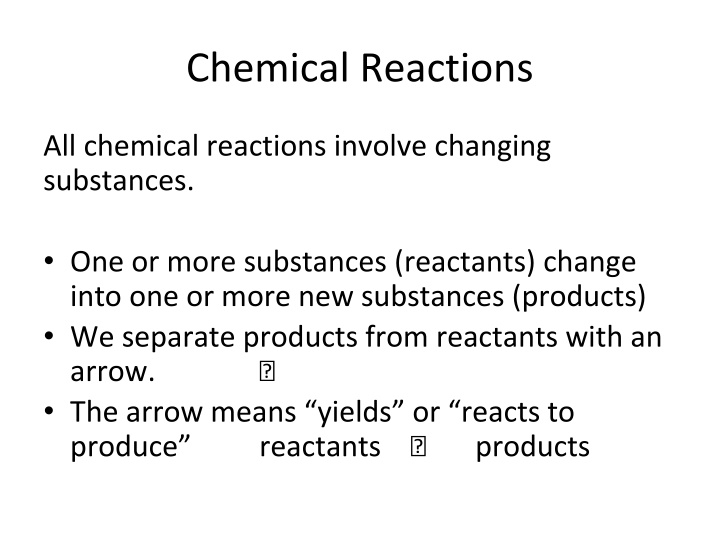
Chemical Reactions All chemical reactions involve changing substances. One or more substances (reactants) change into one or more new substances (products) We separate products from reactants with an arrow. ? The arrow means yields or reacts to produce reactants ? products
How are chemical changes explained? Dalton s atomic theory tells us: in a chemical reaction, the ways atoms are joined together are changed. The atoms themselves are not created or destroyed. The atoms in the products are the same atoms that were in the reactants.
Because of these facts, we can represent chemical reactions as equations. In word equations, such as Iron + oxygen ? iron (III) oxide The reactants are always on the left, the products on the right. In this case, Fe + O2 ? Fe2O3
Fe + O2 ? Fe2O3 What s wrong with this equation? This equation just shows the formulas of the reactants and products a skeleton equation. Skeleton equations do not indicate the relative amounts of reactants and products.
Information about the physical state of a substance is also important: Fe (s) + O2(g) -> Fe2O3(s) Other symbols include (l) and (aq).
Balancing Chemical Equations Write the correct formula for the reactants and the products. Write the formulas for the reactants on the left and the formulas for the products on the right, with a ? in between. Add a + for more than one product or reactant. Count the number of atoms of each element and/or polyatomic ion. (Polyatomic ions can be considered a separate unit) balance the chemical equation using coefficients for each element.
What is a coefficient? A small whole number that appears in front of a chemical formula in a reaction. Check each atom or polyatomic ion to make sure the equation is balanced. Check to see if the coefficients are in the lowest possible ratio.
Why is balancing necessary? The law of conservation of mass must be maintained. In a balanced equation, each side has the same number of atoms of each element.
Examples of Balancing Equations Zinc reacts with oxygen to produce zinc oxide. Zn(s) + O2(g) ? ZnO(s) skeleton Potassium chlorate is heated, decomposing into potassium chloride and oxygen gas KClO3(s) ? KCl(s) + O2 (g)
Types of Chemical Reactions 2Zn(s) + O2(g) ? 2ZnO(s) AB A + B Making a compound from two elements (or two compounds) is called a synthesis reaction ? 2KClO3(s) ? AB The breaking apart of a compound into elements and/or compounds is called a decomposition reaction 2 KCl(s) + 3 O2 (g) A + ? B
Two Other Types of Reactions Single Replacement K(s) + H2O (l) ? KOH(aq) + H2(g) Double Replacement KI(aq) + Pb(NO3)2(aq) ? KNO3(aq) + PbI2(s)
Zn + HCl ? ZnCl2 + H2 NaClO3 ? NaCl + O2 P4 + Cl2 ? PCl3 HCl + Mg(OH)2? MgCl2 + H2O BaO + SO3 ? BaSO4 Pb + AgNO3 ? Ag + Pb(NO3)2 AgNO3+ Na2CrO4 ? Ag2CrO4+ NaNO3
Al + Fe3O4? Al2O3 + Fe NO2 ? NO + O2 Na3N ? Na + N2 Pb(NO3)2 + KI ? KNO3 + PbI2 CaO + CO2 ? CaCO3 MgCO3? MgO + CO2 HNO3+ Mg(OH)2 ? Mg(NO3)2 + H2O
Ca + H2O ? Ca(OH)2 + H2 Fe + O2 ? Fe2O3 Cl2 + KI ? KCl + I2 Ba(NO3)2 + Na2SO4 ? BaSO4+ NaNO3 Ag2O ? Ag + O2
More Equations Fe + O2? Fe2O3 P4O10+ H2O ? H3PO4 C3H8+ O2 ? CO2 + H2O HgO ? Hg + O2 SrBr2+ (NH4)2CO3? SrCO3+ NH4Br Al + O2? Al2O3 Al + AgNO3? Al(NO3)3 + Ag
Al + HCl ? AlCl3+ H2 F2+ HCl ? HF + Cl2 Au2O3 ? Au + O2 NH4NO3? N2O + H2O H2SO4+ NaOH ? Na2SO4+ H2O Xe + F2? XeF6 Ca(ClO)2? CaCl2 + H2O
Fe + H2O ? Fe2O3 + H2 Fe2(SO4)3+ KOH ? K2SO4 + Fe(OH)3 N2+ O2? N2O Mg + CuCl2? MgCl2 + Cu KClO3? KClO4+ KCl K2O + H2O ? KOH I4O9? I2O6 + I2 + O2 This last one has many possible solutions!!!
Mother of All Equations Joined: 18 Nov 2006 Posts: 1 Posted: Sat Nov 18, 2006 8:47 am Post subject: "mother of all equations" Hello, I need a some help with homework. My teacher considers this the "mother of all equations" and she gave it to us to solve... I need to balance the following equation, charges included, this reaction takes place in an aqueous solution. [Cr(N2H4CO)6]4[Cr(CN)6]3 + KMnO4 + H2SO4 =(this is an arrow)= K2Cr2O7 + MnSO4 + CO2 + KNO3 + K2SO4 + H2O