Understanding Chert and Siliceous Sediments: Formation and Varieties
Explore the composition, formation, and varieties of chert and siliceous sediments, including flint, jasper, chalcedony, and opal. Learn about the role of silica in generating hydrocarbons in sedimentary rocks.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
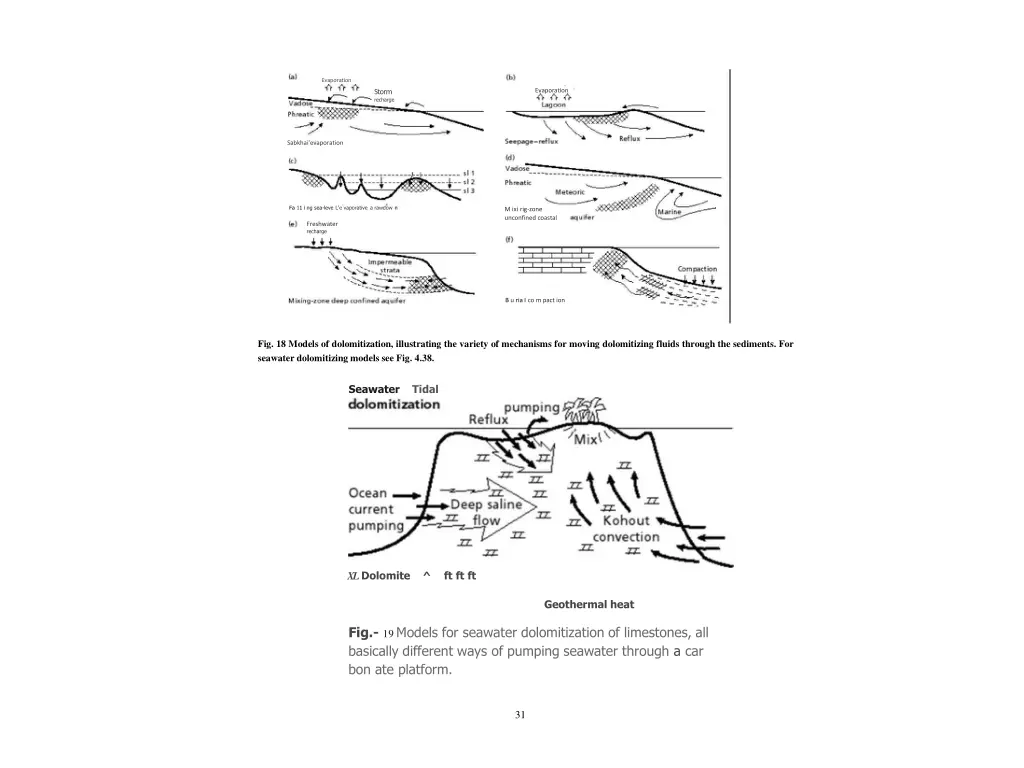
Evaporation Evaporation Storm recharge Sabkhai'evaporation Fa 11 i ng sea-leve L'e vaporative a rawdow n M ixi rig-zone unconfined coastal Freshwater recharge B u ria I co m pact ion Fig. 18 Models of dolomitization, illustrating the variety of mechanisms for moving dolomitizing fluids through the sediments. For seawater dolomitizing models see Fig. 4.38. Seawater Tidal XL Dolomite ^ ft ft ft Geothermal heat Fig.- 19 Models for seawater dolomitization of limestones, all basically different ways of pumping seawater through a car bon ate platform. 31
FABRIC -SELECTIVE NON-FABRIC-SELECTIVE 1 1 Fracture FR FABRIC-SELECTIVE OR NOT Interparticle BP Fenestral FE Breccia BR r r/ V' Boring BO IT S # ^ Intraparticle WP Fig. 20 Porosity types Based on Chouette and Pray (1970) WJ M it Shelter SH Channel CH IS? ill 32
8. Chert and Siliceous Sediments Chert is composed of microcrystalline, cryptocrystalline and microfibrous quartz, chalcedony and opal. Chert is usually hard, dense, and white or black (flint) and red (jasper), with conchoidal fracture. Where it occurs in chalk or marl, it is usually called flint Two varieties of chert are distinguished: bedded and nodular. Most bedded cherts are found in relatively deepwater successions and develop from deposits of siliceous shells of microscopic organisms, such as radiolaria and diatoms oozes. Some bedded cherts are associated with pillow lavas with volcanic associations. Nodular cherts are common in limestones by diagenetic replacement. Flint is a popular name for chert nodules occurring in Cretaceous chalks. Silica for many chert nodules is derived from dissolution of sponge spicules or siliceous plankton. Some nodular chert precipitates from pore fluids, particularly in carbonate rocks. The organic matter of radiolarians and diatoms, another important source of many sedimentary rocks for hydrocarbons generation.. The decay of these materials at in deeply buried sedimentary rocks produces oil, natural gas, and coal. There are numerous varieties of chert, classified based on their visible, microscopic and physical characteristics. Some of the more common varieties are: Flint is a compact microcrystalline quartz. It is found in chalk or marly limestone formations and is formed by a replacement of calcium carbonate with silica. It is commonly found as nodules. This variety was often used in past times to make bladed tools. Jasper is a variety of chert formed as primary deposits, found in or in connection with magmatic formations which owes its red color to iron) inclusions. Jasper frequently also occurs in black, yellow or even green (depending on the type of iron it contains). Jasper is usually opaque to near opaque. Chalcedony is a microfibrous quartz. Agate is distinctly banded chalcedony with successive layers differing in color or value. 33
Opal is a hydrated silicon dioxide. It is often of a Neogenic origin. In fact is not a mineral (it is a mineraloid) and it is generally not considered a variety of chert, although some varieties of opal (opal-C and opal-CT) are microcrystaline and contain much less water (sometime none). Often people without petrological training confuse opal with chert due to similar visible and physical characteristics. Porcelanite is a term used for fine-grained siliceous rocks with a texture and a fracture resembling those of unglazed porcelain. Siliceous sinter is porous, low-density, light-colored siliceous rock deposited by waters of hot springs and geysers. Source and Origin of cherts The alternative views are: 1- that the cherts are entirely biogenic in origin through plankton blooms. From the biogenic amorphous opal, referred to as opal-A, the first diagenetic stage is the development of crystalline opal, referred to as opal-CT, also called disordered cristobalite, alpha-cristobalite, that replaces radiolarian and diatom skeletons and is precipitated as bladed crystals forming microspherules (5-10 um called lepispheres). Further diagenesis results in the opal-CT being converted to microquartz crystals but also chalcedonic quartz. This recrystallization of opal-CT to quartz obliterates the structure of many diatom and radiolarian. The formation of chert from opal-CT has been referred to as a maturation process. The term porcelanite or opaline-claystone is also used for the metastable precursor to chert. 2- that the cherts are a product of submarine volcanism A better understanding of submarine volcanism in recent years, through plate tectonic theory, has made a volcanic-sedimentary origin of cherts less likely. 3- Directly through inorganic precipitation of silica gel derived from sea water, subaqueous magmas or indirectly through plankton blooms, 4- Solubility of pre-existing silicate rocks through pH changes >9 and reprecipitation of silics where pH is <9 5- Deposition of silica by waters of hot springs and geysers such as siliceous sinter. 6- Silica could also produce from diagenesis of shale and alteration of feldspars. 9. Evaporites 34
Evaporite is a name for a water-soluble mineral sediment that results from concentration and crystallization by evaporation from an aqueous solution.-111 There are two types of evaporate deposits: marine, which can also be described as ocean deposits, and non-marine, which are found in standing bodies of water such as lakes. Formation of evaporite rocks All water bodies on the surface and in aquifers contain dissolved salts, the water must enter a restricted environment where water input into this environment remains below the net rate of evaporation. This is usually an arid environment with a small basin fed by a limited input of water. When evaporation occurs, the remaining water is enriched in salts, and they precipitate when the water becomes supersaturated. When scientists evaporate ocean water in a laboratory, the first phase begins when about 50% of the original water depth remains. At this point, minor carbonates begin to form. The next phase is left with about 20% of its original level. At this point, the mineral gypsum begins to form, which is then followed by halite at 10%. The most common minerals that are generally considered to be the most representative of marine evaporates are anhydrite, gypsum, sylvite, and halite, carnallite, polyhalite, and kainite. Kieserite (MgSO4) may also be included, which often will make up less than four percent of the overall content. Evaporite Minerals The minerals precipitate out of solution in the reverse order of their solubilities, such that the order of precipitation from sea water is Calcite (CaCO3) and dolomite (CaMg(CO3)2) Gypsum (CaSO4-2H2O) and anhydrite (CaSO4). Halite (i.e. common salt, NaCl) Potassium and magnesium salts Gypsum is a calcium sulfate mineral containing water of hydration. Primary gypsum occurs as elongate crystals of selenite Gypsum in the form of colorless clear crystals. when it forms from precipitation out of water. If it forms as a result of the rehydration of anhydrite it has a fine crystalline form in nodules of alabaster. Gypsum also occurs as a fibrous form in secondary veins. Gypsum is readily distinguished from calcium carbonate minerals in the field because it is softer (hardness 2, easily scratched with a finger nail) and does not react with dilute HCl: it can be distinguished from halite by the fact that it does not taste salty. Crystals of gypsum have a low relief when they are viewed under the microscope, cleavage is usually well developed and the birefringence colours are low-order greys. Anhydrite is a harder (hardness 3.5), denser mineral than gypsum: it is commonly white in hand specimen, and is not easily scratched by a fingernail. In thin section the high density means crystals have a relatively high relief; birefringence colours are moderate, higher-order colours than gypsum. Non-marine evaporites Non-marine deposits may also contain halite, gypsum, and anhydrite, and may in some cases dominated by these minerals, although they did not come from ocean deposits. Saline lakes includes things such as perennial lakes, which are lakes that are there year-round, playa lakes, which are lakes that appear only 35
during certain seasons. Common minerals that are found in these deposits include blodite, borax, epsomite, , glauberite, mirabilite,thenardite and trona Textures of evaporate minerals Nodular and enterolithic textures are typical of anhydrite precipitated in a marine sabkha (supratidal) environment, so that other peritidal sediments may be interbedded (e.g., microbial laminites/stromatolites, fenestral lime mudstones/dismicrites), or in a continental sabkha, where fluvial and aeolian sediments may be associated. Beds of gypsum may also consist of large (up to a metre or more) twinned crystals (selenite), normally arranged vertically. This type of gypsum is typical of shallow-subaqueous precipitation. Gypsum interlaminated with organic matter or calcite is typical of subaqueous (deeper water) precipitation. Most ancient gypsum exposed at the surface is actually secondary gypsum (alabastrine gypsum) formed by the replacement of anhydrite or primary gypsum or veins of fibrous gypsum (satin spar). The lozengeorlenticular l shapes of gypsum crystals are distinctive. Halite pseudomorphs are readily identified by their cubic shape and hopper crystal form. Economic importance of evaporates Evaporites are excellent indicators of paleoclimate: it takes a hot and arid climate for major evaporite deposits to form. Evaporite minerals, especially nitrate minerals, are economically important in Peru and Chile for using in the production on fertilizer and explosives. Thick halite deposits are used for the disposal of nuclear waste. Halite formations are famous for their ability to form diapirs, which produce ideal locations for trapping petroleum deposits. Evaporites can form seals for the accumulation of petroleum hydrocarbons in reservoir rocks. Origin of evaporites Evaporites are chemically precipitated from restricted bodies of sea water, lakes, lagoons, marine embayments, grabens and intracratonic rifts (faulted bounded basins). Some evaporites are precipitated in non-marine environments such as salt lakes and inland sabkhas. Others precipitated in marine environments such as coastal sabkhas and deep barred basins. The principal modes and depositional environments of evaporite from a shallow to deep standing body of water are given below. The deep marine barred-basin and desiccated basin models explain occurrence and development of thick evaporate sequences, whereby a water body partially isolated from the open ocean is subjected to net evaporation (evaporation is greater than fresh-water runoff into the basin). Some evaporite deposits are relatively thin and intimately associated with sediments of other compositions, and show excellent evidence of very shallow-water conditions, like desiccation cracks. An appealing way of accounting for such evaporites is to assume that they are sabkha deposits. A sabkha is a low-lying but supratidal surface along a coast with net evaporating conditions and little supply of siliciclastic sediment. 36