Understanding Conductometry: Principles and Applications
Conductometry is an electrochemical analysis method based on measuring the electrical conductivity of a solution. It relies on the ability of substances to conduct electrical current, with various factors affecting conductivity. Discover the advantages, disadvantages, and types of electrolytes in this comprehensive guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
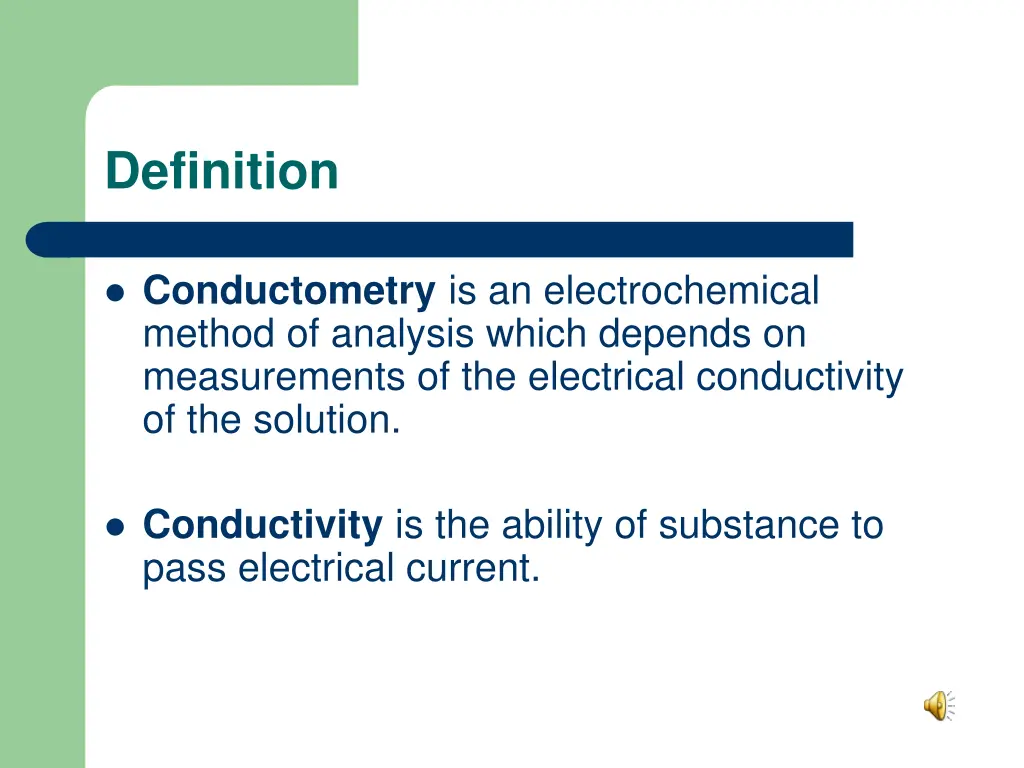
Definition Conductometry is an electrochemical method of analysis which depends on measurements of the electrical conductivity of the solution. Conductivity is the ability of substance to pass electrical current.
How current flow in solution In metallic conductors, electricity is carried out by electrons However, conduction of electricity through an electrolyte solution involves migration of positively charged species towards the cathode and negatively charged species towards the anode. All ions in the solution contribute to electrical conductivity, therefore this method is considered a bulk method.
Electrochemical cell Voltage source Analyte solution Anode Cathode
Advantages and disadvantages Advantages: 1. Conductivity is the simplest electrochemical method that needs simple equipments. 2. The measurement is easy. 3. It has high degree of precision. 4. It is suitable for both colored and turbid solutions. Disadvantages: 1. Non selective as all ions contribute to conductivity of the solution.
What is a conductive solution? Conductivity is typically measured in aqueous solutions of electrolytes. Electrolytes are substances containing ions, i.e. solutions of ionic salts or of compounds that ionize in solution. The ions formed in solution are responsible for carrying the electric current. Electrolytes include acids, bases and salts and can be either strong or weak. Most conductive solutions measured are aqueous solutions, as water has the capability of stabilizing the ions formed by a process called solvation.
Strong and weak electrolytes Strong electrolytes are substances that are fully ionized in solution such as ionic solids and strong acids, for example HCl. Solutions of strong electrolytes conduct electricity because the positive and negative ions can migrate largely independently under the influence of an electric field. Weak electrolytes are substances that are not fully ionized in solution. For example, acetic acid partially dissociates into acetate ions and hydrogen ions, so that an acetic acid solution contains both molecules and ions.
Factors affecting the conductivity of the solution: Concentration of the ions in the electrolyte solution. Valence of ions; A2-will carry twice as many electrons as A1-. Ca2+ shows more conductance values at infinite dilution compared to Na+. Mobility of ions: Highly mobile ions will carry more electrical charges and hence have high conductivity. Mobility is controlled by the viscosity and temperature of the solvent the size of ion and the voltage applied. In general, solvent of low viscosity have higher mobility and also increase by raising the temperature. Small size ion shows high value due to its higher mobility. Temperature: Conductivity increase by increasing the temperature.
Some important laws, definitions and relations Ohm's Law : It is applied by metallic conductor as well as electrolytic solution. It slates that the current (i) in amperes flowing in a conductor is directly proportional to the applied electromotive force (E) in volts and inversely proportional to the resistance (R) in ohms of the conductor:
Conductance Conductance (G) is the reciprocal of the resistance. Conductance = 1/R; this is measured in reciprocal ohms (or -1), for which the name Siemens (S) is used. Conductance is directly proportional to the cross sectional area in square centimeters (a), and is inversely proportional to the length, (L) in cm. Where, is a proportionality constant called specific conductance. Since, = L/R a, thus its units = cm/cm2 = cm-1 -1. Specific conductance increases by increasing the concentration of the solution.
Molar conductance () Molar conductance ( ) of an electrolyte is defined as the conductivity of one mole of the substance or it is the value of contributed by one mole of the ions contained in 1000 cm3of solvent. Where, is the specific conductance, C is the molar concentration. 1000 is number of mole per milliliters Units : l = k (1000/C) = -1cm-I(1000/mol) = -1cm-Imol-1 The molar conductivity of strong electrolytes increases by dilution.
Limiting ionic molar conductivity As it is mentioned before, the molar conductivity increases by dilution, but it appears to approach a limiting value known as the molar conductivity at infinite dilution; . At infinite dilution the ions are independent of each other, and each contributes its part of the total conductivity. The Limiting ionic molar conductivity is constant and specific for each ion. Table 1, shows the values for the limiting ionic molar conductivities for some ions in water at 25 C.
Limiting ionic molar conductivity at 25 C ( cm2 -1mole-1) Cation Molar conductance ( -1cm-Imol-1) 50 62 74 74 106 106 107 108 119 205 350 Anion Molar conductance ( -1cm-Imol-1) 45 52 61 71 76 77 78 119 160 197 240 Na+ Ag+ K+ NH4+ Zn2+ Mg2+ Cu2+ Fe2+ Ca2+ Fe3+ H+ HCO3- HSO4- MnO4- NO3- Cl- I- Br- CO32- SO42- OH- PO4-3
Measurement of conductivity Conductivity cell: Temperaturecontrol An alternating rather than direct current is used The electrodes of conductivity cells are usually made of inert material
Wheatstone bridge With fixed resistances, R1and R2, the variable resistance Rv; is adjusted until no current flows through the galvanometer. Under these conditions: R1X Rv= R cellX R2 This gives a value for resistance of the cell which can be converted to conductance by calculating the reciprocal.
APPLICATIONS OF POLAROGRAPHY direct conductimetric measurements Purity of water The control of boiler feed water Environmental application In ion chromatography
APPLICATIONS OF POLAROGRAPHY CONDUCTIMETRIC TITRATION When two electrolyte solutions that do not react with each other are mixed, provided that there is no appreciable change in volume, the conductance of the solution will rise because of the increase in the numbers of ions. However, if there is a reaction between the ions, one ion being replaced by another, the conductance of the solution will alter depending upon the relative mobilities of the ions involved
Titration curve The conductance is plotted against the volume of the reagent, to give a graph consisting of two straight lines intersecting at the equivalence point. The titrating reagent which is 20 to 100 times more concentrated than the solution being titrated A correction for the dilution effect may, however, be made by multiplying the values of the conductance by the factor ( V + v)/ V measurements near the equivalence point have no special significance
Advantages of conductimetric titration methods: It may be applied where visual or potentiometric methods fail to give results owing to considerable solubility or hydrolysis at the equivalence point, The method is as accurate in dilute as in more concentrated solutions. It can also be employed with colored solutions. It may be noted that very weak acids, such as boric acid and phenol,. Mixtures of certain acids can be titrated more accurately by conductimetric than by potentiometric (pH) methods.
Limitation and necessary precaution in conductimetric titrations : temperature control, in conductance measurements. Effect of change of conductance of the solution during the reaction and upon the addition Conductimetric titration is limited to comparatively simple systems in which there are no excessive amounts of reagents present. (oxidation titrations ) Conductimetric titrations have been largely superseded by potentiometric procedures, but there are occasions when the conductimetric method can be advantageous. 1. 2. 3.
Examples for conductimetric titrations Neutralization titrations: a) Titration of Strong acid with a strong base b) Titration of Strong acid with a weak base c) Titration of Weak acid with a strong base d) Titration of Weak acid with a weak base e) Titration of mixture of strong and weak acids with either strong or a weak base: f) Displacement titrations: Precipitation titrations:
Neutralization titrations: a) Titration of Strong acid with a strong base Upon titration of aqueous solution of acid HCl with standard NaOH, the reaction can be expressed as follows: (H++ Cl-) + (Na++ OH -) H2O + (Na++ Cl-) 350 76 50 198 zero + 50 76 Thus, conductance associated with hydrogen ion starts at a value proportional to its molar conductivity ( 350, and decreases linearly to zero at the equivalence point due to formation of water by the added OH- ( 198). Hydroxide ion is not present in significant amounts until the equivalence point is passed. Then the conductance associated with this ion increases linearly to its molar conductivity value ( 198, when a 100% excess of the titrant is added.
a) Titration of Strong acid with a strong base the shape of the titration curve
b) Titration of Strong acid with a weak base
c) Titration of Weak acid with a strong base be neglected. Conductance OH- mL of NaOH
d) Titration of Weak acid with a weak base Titration of weak acid (acetic acid) with weak base (NH4OH)
e) Titration of mixture of strong and weak acids with either strong or a weak base Titration of a mixture of strong acid and weak acid (acetic acid) with strong base or weak base (NH4OH)
2. Precipitation titrations Consider the titration of sodium chloride with silver nitrate (Fig. 3.7). The reaction equation for the present conductimetric titration may be presented as follows: (Na++ Cl-) + (Ag++ NO3-) AgCl + (Na++ NO3-) 50 76 62 71 50 71 Titration of sodium chloride with silver nitrate