Understanding Diffusion Mechanisms in Materials Science
Explore the concept of diffusion in materials science, including interdiffusion, self-diffusion, and different diffusion mechanisms like vacancy diffusion and interstitial diffusion. Learn about the importance of steady-state diffusion and diffusion rate calculations. Discover the process of material transport by atomic motion and how atoms diffuse in metals over time.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
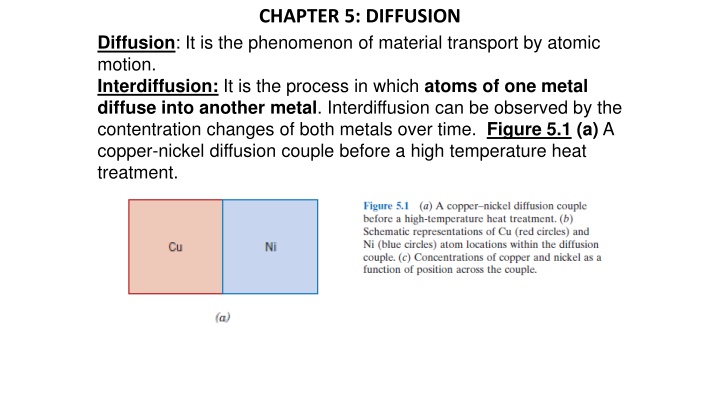
CHAPTER 5: DIFFUSION Diffusion: It is the phenomenon of material transport by atomic motion. Interdiffusion: It is the process in which atoms of one metal diffuse into another metal. Interdiffusion can be observed by the contentration changes of both metals over time. Figure 5.1 (a) A copper-nickel diffusion couple before a high temperature heat treatment.
CHAPTER 5: DIFFUSION Figure 5.2 (a) A copper-nickel diffusion couple after a high temperature heat treatment, showing the alloyed diffusion zone. (c) Concentration of copper and nickel as a function of position across the couple after the heat treatment.
CHAPTER 5: DIFFUSION Self-diffusion: If diffusion of atoms occurs in pure metals, this diffusion is called self-diffusion. Self-diffusion cannot be observed by concentration changes because there is only one type of atom in a pure metal which diffuses in the metal.
DIFFUSION MECHANISMS A. Vacancy Diffusion: In vacancy diffusion, an atom from a normal lattice position moves to a nearby vacant lattice site. Figure 5.3 (a)kafes
DIFFUSION MECHANISMS B. Interstitial (Arayer) Diffusion: In this diffusion mechanism, an atom from an interstitial position moves to a nearby interstitial position that is empty. Figure 5.3 (b) The atoms that are diffusing in this mechanism are usually small impurities such as hydrogen, nitrogen and oxygen in the matrix.
STEADY-STATE DIFFUSION Diffusion is a time-dependent process. It is often neccessary to know the diffusion rate. Diffusion rate: It is the amount of material that diffuses in a certain time. Diffusion rate is often expressed as a Diffusion flux (J). Diffusion flux (ak ): It is the mass or number of atoms (M) that are diffusing through a cross-sectional area (A) of a solid per unit time. The unit of J is kgm-2s-1. M 1 M = = J J : in differenti al form At A t
If the diffusion flux does not change with time, this is called steady-state (kararl yay nma) diffusion. J = Thin metal plate C D Fick s First Law x D: The diffusion coefficient Its unit is m2s-1 Direction of diffusing atoms x- direction C: Concentration, kgm-3 Cross-sectional Area, A
FACTORS THAT INFLUENCE DIFFUSION 1. Diffusing atoms: Different materials have different Diffusion coefficients (D). If you change the type of material, then it influences diffusion. 2. Temperature: Diffusion coefficient increases with Temperature. So, if you increase the Temperature, then the diffusion coefficient increases.
