Understanding Enthalpy of Formation vs. Enthalpy of Combustion in Chemistry
Explore the differences between enthalpy of formation and enthalpy of combustion in chemistry under standard conditions. Dive into the significance and applications of both concepts to gain a deeper understanding of thermochemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
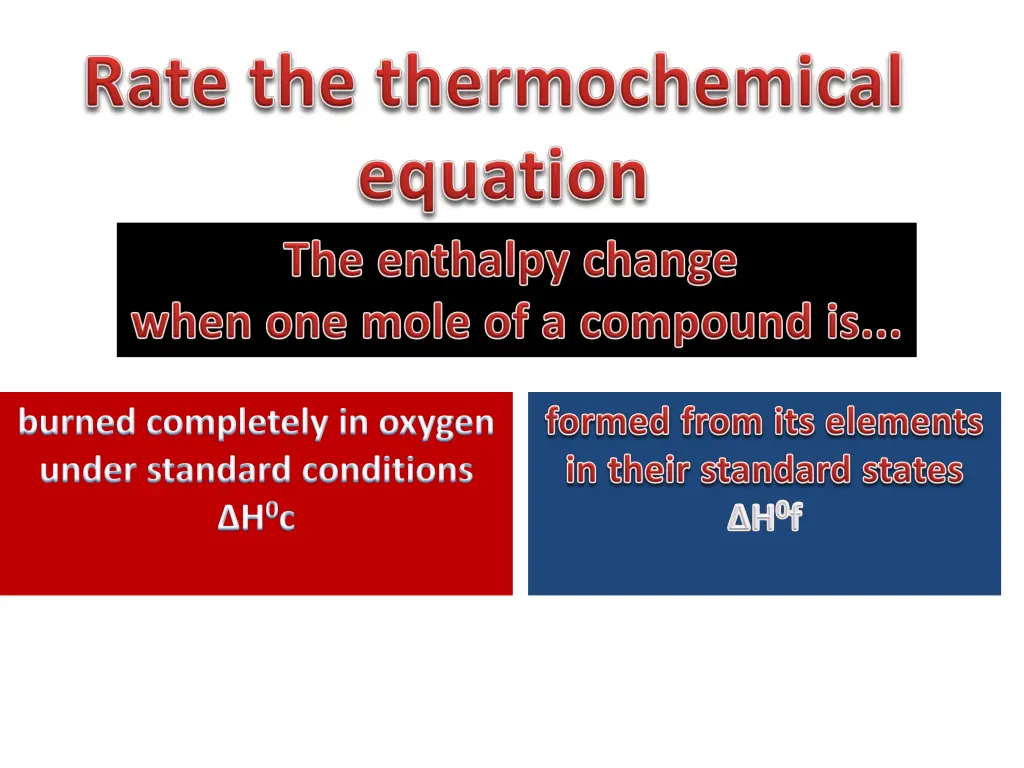
burned completely in oxygen under standard conditions H0c
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
Enthalpy of formation or Enthalpy of combustion Both Neither
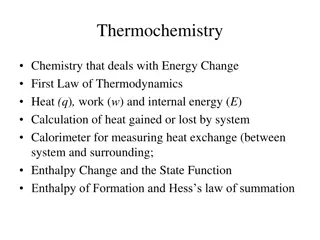
Hess's Law Section 15-4 Hess s law The enthalpy change accompanying a chemical change is independent of the route by which the chemical change occurs.
Hess's Law (cont.) Section 15-4
Write a balanced equation to represent A. the enthalpy of formation of butane. B. the enthalpy of combustion of butane