Understanding IR Spectroscopy in Chemical Analysis
IR spectroscopy is a powerful technique used in chemical analysis to identify and characterize chemical substances based on their interaction with infrared light. This method provides valuable information about molecular structure and functional groups present in a sample, making it indispensable in various fields such as pharmacognosy. Learn about the principle, instrumentation, and diverse applications of IR spectroscopy in pharmaceutical analysis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
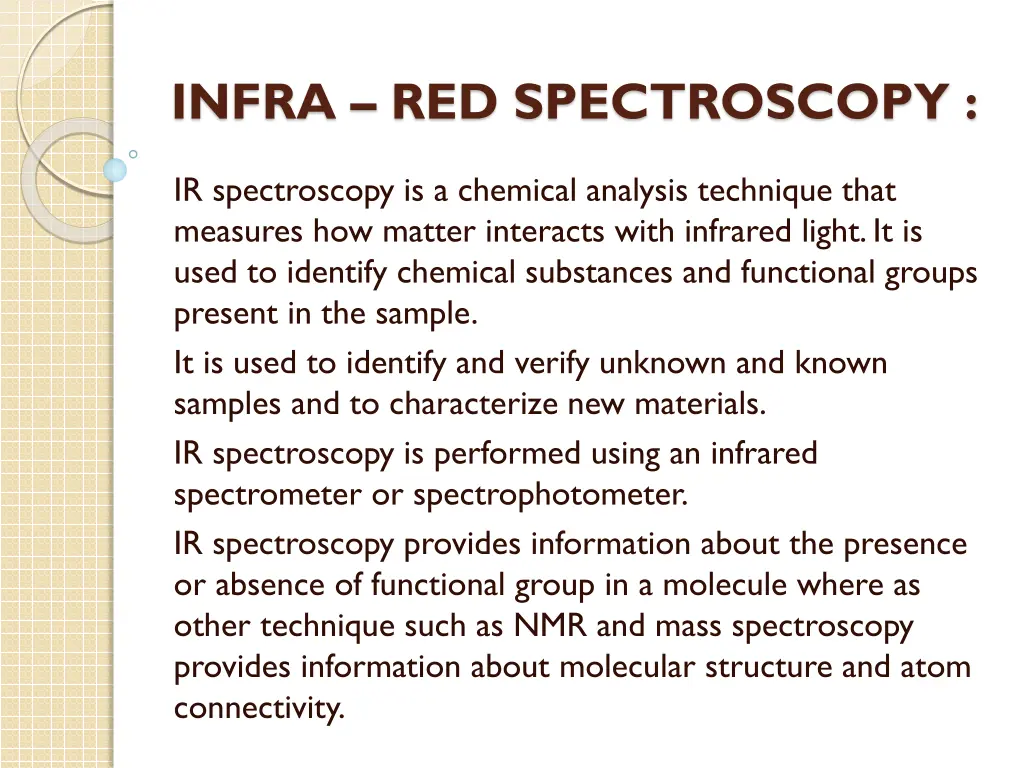
INFRA RED SPECTROSCOPY : IR spectroscopy is a chemical analysis technique that measures how matter interacts with infrared light. It is used to identify chemical substances and functional groups present in the sample. It is used to identify and verify unknown and known samples and to characterize new materials. IR spectroscopy is performed using an infrared spectrometer or spectrophotometer. IR spectroscopy provides information about the presence or absence of functional group in a molecule where as other technique such as NMR and mass spectroscopy provides information about molecular structure and atom connectivity.
PRINCIPLE : The principle of infrared spectroscopy is that molecules absorb specific light frequencies that are characteristic of their structure. When a beam of infrared light passes through a sample it interacts with the chemical bonds in those molecules to vibrate. The type of IR wavelength absorbed by a molecule depends on the type of the atom and chemical bond present in the molecule. The infrared range covers 700 1000nm wavelength or 14,286 12,800 cm-1
APPLICATIONS : Infrared (IR) spectroscopy has many uses in pharmacognosy, including: Drug identity:IR spectroscopy can verify the identity of drugs. Purity:IR spectroscopy can test the purity of drugs. Structural investigations:IR spectroscopy can help determine the structural formula of drugs.For example, IR spectroscopy was used to decipher the structural formula of penicillin during World War II. Crystalline structures: IR spectroscopy can help determine the crystalline structures of drugs. Interactions between active medicaments and excipients: IR spectroscopy can help determine how active medicaments interact with excipients. Contaminants: IR spectroscopy can quickly test samples for unwanted contaminants. Organic compounds: IR spectroscopy can test for organic compounds, such as proteins.