Understanding Methane: Structure, Properties, and Reactions
Explore the hybridization process for the true structure of methane, its physical properties, and reactions, along with its significance as a major component of natural gas. Learn about the non-polar nature of methane, its formation, and how it reacts under different conditions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
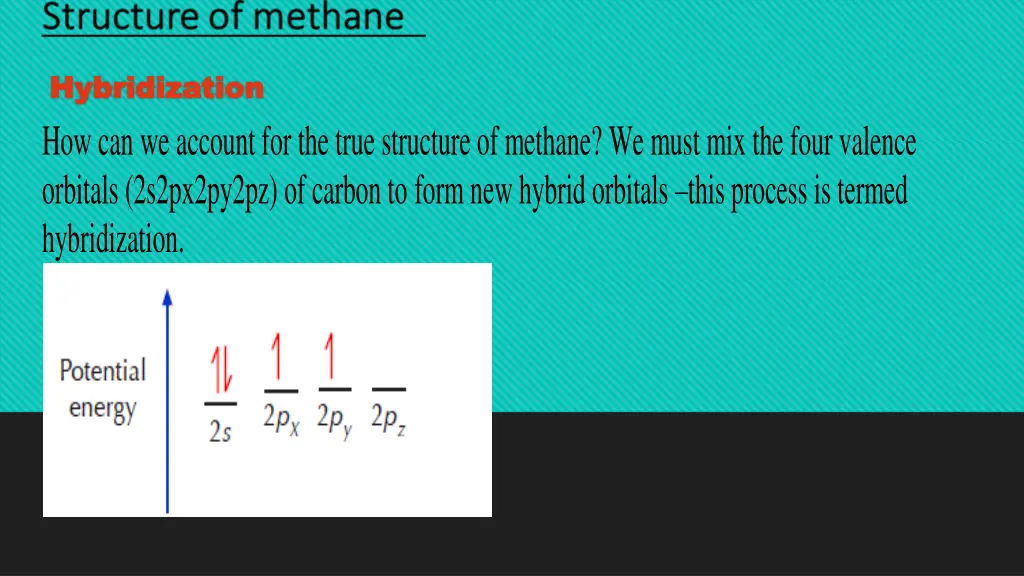
Hybridization Hybridization How can we account for the true structure of methane? We must mix the four valence orbitals (2s2px2py2pz) of carbon to form new hybrid orbitals this process is termed hybridization.
In order to achieve the octet rule, carbon must use its 4 valence electrons when bonding to other atoms. However, only unpaired electrons can bond. That means that the two paired electrons occupying the 2s orbital must become unpaired before they can bond. Since the energy gap between the 2s and 2p orbitals is very small, one of the 2s electrons can be promoted to the empty 2p orbital, leading to the following situation:
The process that leads to their formation is called sp3 hybridization All four sp3 orbitals that result from this process are equivalent. That means that they have the same size, shape, and energy. According to VSEPR (valence shell electron pair repulsion) theory, such orbitals will orient themselves in 3-D space to be as far apart from each other as possible. The resulting shape is then a tetrahedron, where the carbon nucleus is at the center and the orbitals point to the corners of the tetrahedron
Physical properties of methane Methane is non-polar compound. Attraction between non-polar molecules is van der Waals forces, which is weak interaction compared with ionic attraction between sodium and chloride ions. Methane is colorless gas, when liquefied is less dens than water. Slightly soluble in water, but very soluble in organic liquids as gasoline, ether and alcohol.
Source Source Methane is product of an aerobic (without air) decay of plant, breakdown of certain very complicated molecules. Methane is the major constituent of natural gas (up to 97%).
Reactions of methane Because of the inert nature of methane, therefore, it reacts only with the highly reactive substance or under very vigorous conditions. 1-Oxidation, Heat of combustion Methane like other hydrocarbons subjected to combustion (burning of natural gas) by flame to form carbon dioxide and water. The quantity of heat released (213 Kcal/mol).
Heat of combustion, the quantity of heat evolved when one mole of a hydrocarbon is burned to carbon dioxide and water, for methane its value is 213 kcal. * By thecontrolled, high temperature, partial oxidation of methane give acetylene and hydrogen.
Chlorination may continue to yield CHCI3,trichloromethane (chloroform) then, CC14tetra chloromethane ( carbon tetrachloride). In general (Halogenation) X2 X2 X2 X2 CX4+HX CH2X2 + HX CHX3 + HX CH3X + HX CH4 Reactivity is F2> Cl2 > Br2 > I2
There are two major types of stereoisomer: conformational isomers and configurational isomers. Configurational isomers include optical isomers, geometrical isomers, enantiomers and diastereomers.
Conformational isomers Atoms within a molecule move relative to one another by rotation around single bonds. Such rotation of covalent bonds gives rise to different conformations of a compound. Each structure is called a conformer or conformational isomer. Generally, conformers rapidly interconvert at room temperature. Conformational isomerism can be presented with the simplest example.
