Understanding Neutralization Titrations and Standardization Methods
"Learn about neutralization titrations, the standardization process for solutions, primary standard materials, acid-base reactions, titration procedure, and the importance of indicators in laboratory techniques." (264 characters)
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
NEUTRALIZATION NEUTRALIZATION TITRATIONS TITRATIONS Standardization of HCl and NaOH solutions
TITRATION / STANDARD SOLUTION / PRIMARY STANDARD Titration is a laboratory technique that measures the concentration of an analyte using the reaction between the analyte and standard solution. Standard solution (titrant) is a solution containing an accurately known concentration. (X.XXXX M) Last week you prepared solutions with approximate concentration, because NaOH and HCl cannot be obtained in a sufficient pure state. Now you need to determine the exact concentration (molarity) of those solutions. is the process of determining the exact Standardization concentration (molarity) of a solution. We use primary standard materials to standardize a solution. A reaction between primary standard material and a solution allows us to determine the the exact concentration of that solution. To standardize a solution We perform titration of the solution and the primary standard material.
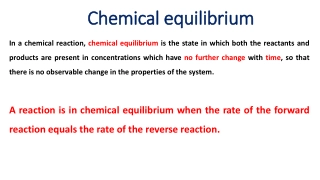
NEUTRALIZATION TITRATIONS The reaction between an acid and a base is called as neutralization reaction. Acid-base titrations are also called neutralization titrations. Acidimetry is the determination of concentration of basic substances by titration with a standard acid solution, and alkalimetry is the measurement of concentration of acidic substances by titration with a standard base solution.
PRIMARY STANDARD MATERIALS Primary standards for acid standardization Na2CO3 (sodium carbonate) TlCO3(talium carbonate) KHCO3 (potassium bicarbonate) Primary standards for base standardization H2C2O4.2H2O (oxalic acid dihydrate) KHC8H4O4 (potassium biphthalate) HC7H5O2 (benzoic acid)
The end-point (equivalence point) of acid-base reactions are observed by using indicators which are substances that changes colors near their pKa. Therefore a suitable indicator should be selected for acids and bases that are reacted. A titration curve is a plot of pH vs. the amount of titrant added. Shape of titration curves differ for weak and strong acid-bases or for polyprotic acids and bases. A titration of a polyprotic acid (H3PO4) with a strong base (NaOH) Indicators having pKa in the range of end-points on the titration curves are suitable for that titrations. End-points can also be determined by some other methods such as potentiometry, conductometry, amperometry, spectrophotometry.
TITRATION PROCEDURE Shake solutions well before starting. Put the analyte a flask and add two drops of indicator. Fill the burette with standard solution (titrant) using a beaker. Turn on the valve (stopcock) and let the tip of the burette fill with liquid as well. Check the tip of the buret for leak or air bubble. If there is a leak, assemble the stopcock properly. If an air bubble is present during a titration, volume readings may be incorrect. To remove an air bubble, whack the side of the burette tip while solution is flowing. Read the level of meniscus and take note. Add the titrant drop by drop into the flask while swirling it. Titration must be performed slowly and always holding stopcock with one hand while swirling the flask with other hand.
A white paper under the erlenmeyer flask may help you to determine the end-point.
Standardization of 0.1 N NaOH solution Use indirect weighing (weighing by difference) technique to weigh oxalic acid into erlenmeyer. Carefully weigh 0.1-0.2 gram of oxalic acid (H2C2O4.2H2O) and note the exact amount. Dissolve oxalic acid by adding around 40-50 mL of water into the erlenmeyer flask. Add 1-2 drops of phenolphthalein to the erlenmeyer. Fill the burette with NaOH solution that you want to standardize. Check for leak and bubbles. Read the volume in the burette by looking at bottom of the meniscus. Add NaOH solution drop by drop to the erlenmeyer flask by turning the stopcock while swirling the flask. Continue to the titration until the color of the solution in the flask turns to light pink. Take note the titrant volume you used.
HOW TO WEIGH OXALIC ACID BY INDIRECT METHOD Make sure you have a pencil, a piece of paper and a calculator with you. Take the crucible containing oxalic acid in it from desicator and put it onto a balance. Take a note of the amount on the screen (For example 10.525 g). To take 0.1 - 0.2 g of oxalic acid 10.525 0.2 = 10.325 10.525 0.1 = 10.425 We need to take an amount of oxalic acid that the remaining amount must oxalic acid be in betweeen 10.325 g 10.425 g If the final amount of remaining oxalic acid is 10.352 then we took H2C2O4.2H2O 10.525 10.352 = 0.173 gram MW:126.1 g/mol By using a spatula, take required amount of oxalic acid and transfer it to the flask and keep the spatula in the flask for flushing the oxalic acid left on the surface of the spatula.
Standardization of 0.1 N HCl solution Use your graduated pipette to transfer 10 mL HCl into a erlenmeyer flask and add 1-2 drops of phenolphthalein. Fill a buret with NaOH solution that you already standardized Titrate until light pink color. Take note the titrant volume CALCULATION HCl + NaOH NaCl + H2O (mole ratio 1:1) nHCl = n NaOH V HCl x MHCl = VNaOH x MNaOH Calculate MHCl and report it to your TA with other results in your bench. REFERENCES Analitik Kimya Pratikleri Kantitatif Analiz (Ed. Feyyaz ONUR), 2014