Understanding Nuclear Forces and Nuclei Properties
Explore the fundamental nuclear properties including nuclear forces, saturation property, and the proton-electron hypothesis. Learn about the nature of nuclear forces and the angular momentum of nuclei. Delve into the failures of the proton-electron hypothesis and the implications of nuclear angular momentum on the hyperfine structure.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
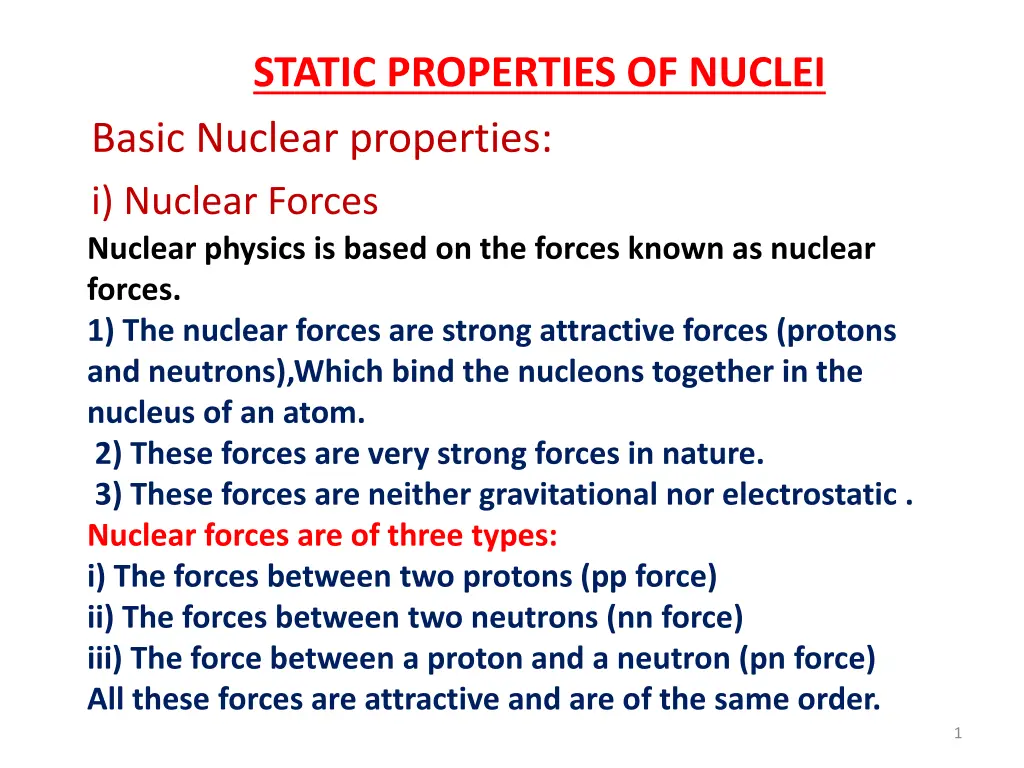
STATIC PROPERTIES OF NUCLEI Basic Nuclear properties: i) Nuclear Forces Nuclear physics is based on the forces known as nuclear forces. 1) The nuclear forces are strong attractive forces (protons and neutrons),Which bind the nucleons together in the nucleus of an atom. 2) These forces are very strong forces in nature. 3) These forces are neither gravitational nor electrostatic . Nuclear forces are of three types: i) The forces between two protons (pp force) ii) The forces between two neutrons (nn force) iii) The force between a proton and a neutron (pn force) All these forces are attractive and are of the same order. 1
ii) Nature of Nuclear Force: Nuclear forces are very strong attractive forces. These strong forces effective only within a very short range of the -15 order of m. 10 These forces are independent of charges. Nuclear forces are limited in range. As a result, each nucleon interacts with only a limited number of nucleons nearest to it. This effect is called saturation of nuclear forces (saturation property) 2
The proton-electron hypothesis: Before the discovery of neutron by Chadwick (1932), it was assumed that nucleus consists of protons and electrons. This concept was arose because: The radioactivity showed that both and particles were ejected from the nuclei of the atoms undergoing transformation and the presence of electrons in the nucleus made reasonable under same conditions, one of them might be ejected. It was also assumed that the -particle could be formed in the nucleus by the combination of four protons and two electrons. It was found that most elements are mixtures of isotopes, and the different isotopes of an element have the same number of protons and the arrangements of electrons, and consequently their spectra have the same general structure. They are distinguished from one another by their different atomic masses(nuclear masses). The isotope effect is not enough to explain the hyperfine structure. The hyperfine structure can be accounted for quantitatively assuming that the nucleus has an angular momentum.(The splitting of a spectral line into a number of lines lying close together, is called hyperfine structure.) 3
The angular momentum of the nucleus; Failure of the proton electron hypothesis: One of the failures of the hypothesis was associated with an unknown property of the nucleus, the angular momentum. The discovery that the nucleus has an angular momentum, or spin which is associated with a magnetic moment was a result of a detailed study of spectral lines. The splitting of a spectral line into a number of lines lying close together, is called hyperfine structure. The properties associated with the hyperfine structure are the mass and angular momentum of the nucleus. 4
i) The isotope effect is not enough to explain the hyperfine structure. The hyperfine structure can be accounted by assuming that the nucleus has an angular momentum . The nuclear angular momentum is a of magnitude h + = I I I(I 1) 2 Where is the quantum number defines greatest possible component of along a specified axis . According to the rule Iz = I h/2 I I The value of I has been found experimentally to depend on the mass number A of the nucleus. If A is even: I is an integer or0 value 0,1,2,3, If A is odd:I is an odd half integral value 1/2, 3/2 ,5/2 , . This rule leads to one of the failures of the proton-electron 5
Example: 7N14 Atomic no. =7 , mass no. = 14 According to p-e hypothesis: atomic number Z contains A-protons and (A-Z) electrons. So 7N14 nucleus would have 14 protons and 7 electrons , and 7 electrons in extra nuclear space. A =14 (even numbers) The contribution of the protons to the angular momentum should be an integral multiple of h/2 . An electron has spin so the contribution of 7 electrons is an odd half integral multiple of h/2 , and the total integral momentum of nitrogen nucleus should be an odd half - integral multiple of h/2 . But the angular momentum of nitrogen nucleus has been found experimentally to be I = 1 , an integer , which is in contradiction to the value obtained by the proton-electron hypothesis. 6
ii) P-e theory also fails to account for the order of magnitude of nuclear magnetic moments. Measurements of the magnetic moments of many nuclei have given the values which are only about 1/1000 of value of the magnetic moment of electron. The magnitude of magnetic moment of electron is, eh 4 = B m c e 20 This quantity is called Bohr magneton All measured nuclear magnetic moments are and their values can be expressed appropriately as, . 0 92 10 10 erg / erg/ gauss gauss 23 eh = = 23 . 0 505 10 / erg gauss N 4 m c H Where, mH is proton mass and N is called the nuclear magneton. Measured value of the nuclear magnetic moment vary from 0 to 5 nuclear magnetons, and the proton has magnetic moment 2.7926 0.0001 nuclear magnetons . Hence, observed mag. moments of nuclei are comparable to that of the proton. If electrons exist inside the nucleus, we should expect to find nuclear magnetic moments must be of the same order of magnitude as that of electron . However, the observed magnetic moments of nuclei are comparable with that of proton. This experimental fact goes against the electrons existing inside the nucleus. 7
III) There is also wave mechanical argument against the existence of free electrons in the nucleus: If an electron exists inside the nucleus, the uncertainty in its position ( x ) may not exceed 10-14 m . According to Heisenberg uncertainty principle, uncertainty in the electrons momentum is, 10 054 . 1 2 x h 34 20 1 1 . 1 10 p kgms 14 10 Approximate momentum of electron, p= 1.1 10-20 kg ms-1. and has K.E. (T) many times greater than its rest energy moc2 i.e. 2 T m c o Hence to find T we can use the relativistic formula , T=pc T 1 , 12 = = 20 8 1 . 1 ( 10 = )( 3 10 ) 3 . 3 10 J 13 6 . 1 10 but MeV J 12 3 . 3 10 = = , 20 63 . So T MeV MeV 13 6 . 1 10 This shows that if an electron exists in the nucleus, the K.E. of the electron must be more than 20 MeV. Electrons of such large energy are never found to be emitted during beta decay. The maximum energy of beta particle emitted is only 2 to 3 MeV. This fact also goes against the existence of electrons inside the nucleus. 8
Nuclear Transmutation and discovery of neutron: Nuclear transmutation is the conversion of one element into another element or one isotope into another. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus is changed. A transmutation can be achieved either by Nuclear reactions (in which an outside particle reacts with a nucleus) or by Radioactive decay where no outside cause is needed. The first artificial transmutation was observed by Rutherford in 1919. He bombarded nitrogen nucleus with alpha particles, the new element formed which was the isotope of oxygen of mass number 17. He + N 2 7 14 4 18 17 1 F O + H 9 8 1 Thus the first transmutation of nitrogen into oxygen was achieved. The protons were identified by magnetic deflection measurements and their energies were considerably greater than those of the bombarding alpha particles. 9
The bombardment with alpha particles was found by Rutherford to cause emission of protons from all the elements of atomic number up to 19 with exception of The most considerable effects occurred with . 1 008 4 . 6 94 12 011 . . 9 012 , , , & H He Li C Be B 1 2 3 6 4 10 . 81 14 007 . 26 98 . , & N Al 4 7 13 After that, Bothe and Becker (in 1930) discovered that Be elements emitted a highly penetrating radiation when alpha particle bambarded, it was thought that its radiation must be a form of gamma ray of very high energy i.e. C He Be + 6 2 4 + h 9 4 13 Curie Juliot (in 1931) found that when the radiation was allowed to fall on substances containing hydrogen, it caused the production of highly energetic particles. Chadwick in 1931 was able to show that the rays emitted from Be, gave rise to rapidly moving atoms when allowed to fall on other substances like He, Li, C,O,N . These results could not be explained that the new radiation consisted of high energetic gamma rays. 10
Chadwick (in 1932) finally proved that the energies of proton ejected from hydrogenous material and of the other rapidly moving atoms could only be explained. The explanation is on the basis that the rays from bambarded Be actually consisted of particles with mass close to that of the proton. These particles produce no tracks in the cloud chamber and not affected by electric or magnetic field. These facts together with the extremely high penetrating power of the particle showed that the charge of the later must be zero. Science new particle was found to be neutral and to have a mass close to 1. Later measurements showed that , the mass of neutron is 1.0098 amu, it is slightly heavier than the proton with mass 1.00458 amu. 11
Hence, James Chadwick( in 1932) discovered that,the atomic nucleus was constituted with neutron and it was a new elementary particle, distinct from the proton. The uncharged neutron of nuclear structure, leading the creation of new radioactive elements ( by neutron irradiation (1934)) and the fission of uranium atoms by neutrons (1938). The discovery of fission led to the creation of both nuclear power and weapons by the end of World War II. Both the proton and the neutron were presumed to be elementary particles until the 1960s, when they were determined to be composite particles built from quarks. 12
It is important to note that the three conservation laws apply to nuclear reactions: i) The charge is conserved, or, the sum of charges on the left is equal to the sum of charges in the right. ii) The number of nucleons in a nuclear reaction is conserved. iii) The mass-energy relation is conserved. e.g. Aluminum is transformed into phosphorous by combining the nucleus with an alpha particle. A neutron is produced as part of the transformation. 13 Al + + 27 4 30 1 He P n 2 15 0 13
The ProtonNeutron Hypothesis: Proton- neutron hypothesis was proposed after the discovery of neutron by Chadwick in 1932. ACCORDING TO THIS HYPOTHESIS, a nucleus of mass no. A and atomic number Z ,contains Z protons and A- Z no. of neutrons inside the nucleus. Z no. of electrons are revolving around the nucleus so as to make atom electrically neutral. The existence of neutron in nucleus satisfies the uncertainty principle. It explains the electron does not pre-exist in the nucleus. The electron is formed just at the instant of emission caused by the transformation of a neutron into a proton as, + (e p n ) And The positron emission is due to the transformation of proton into a neutron as, + (e n p +) The emission of alpha particles from the nuclei of radioactive elements is due to the combination of 2protons and 2neutrons at the instant of emission. Thus this hypothesis explains beta decay and alpha decay. This assumption is also able to explain the observed value of nuclear spin and nuclear magnetic moment. Nuclei having odd mass no. will have half integral spin and those with even mass no. will have integral spin. There is no difficulty in explaining the existence of isotopes. Isotopes of given element differ only in the number of neutrons they contain. 14