Understanding Orbital Symmetry in Cycloaddition Reactions
Explore the fascinating world of orbital symmetry in cycloaddition reactions, where the addition of electron systems leads to bond formations. Learn about stereochemical modes, antarafacial processes, and the correlation between reactant and product orbitals. Discover the applications of the Frontier Molecular Orbital (FMO) method and the Paternò-Büchi reaction in 2+2 and 4+2 cycloadditions. Uncover the intriguing interplay of ground and excited states in thermal and photochemical processes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
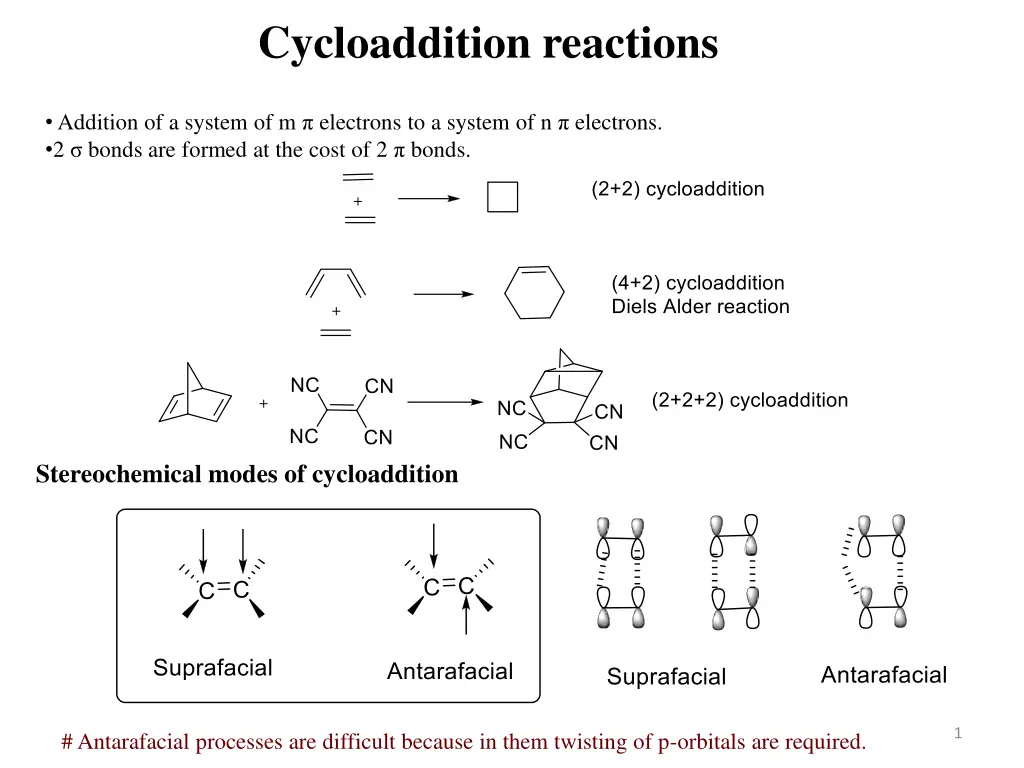
Cycloaddition reactions Addition of a system of m electrons to a system of n electrons. 2 bonds are formed at the cost of 2 bonds. Stereochemical modes of cycloaddition 1 # Antarafacial processes are difficult because in them twisting of p-orbitals are required.
Orbital symmetry in cycloaddition reactions 2+2 cycloaddition 2
Orbital symmetry in cycloaddition reactions Correlation Diagrams h Allowed Forbidden * Ground state orbitals of ethylene correlate with an excited state of cyclobutane. Hence the thermal process is symmetry forbidden. * There is correlation between 1st excited state of ethylene and cyclobutane, The photochemical process is symmetry allowed. 3
Orbital symmetry in cycloaddition reactions (4+2) Cycloaddition reactions h Forbidden Allowed 4 Ground state reactant orbitals are transformed into ground state product orbitals, therefore thermal process is symmetry allowed.
Applications of FMO Method 2+2 Cycloaddition 5
Applications of FMO Method 4+2 Cycloaddition 6
Applications of PMO Method 2+2 Cycloaddition 4+2 Cycloaddition 7