Understanding pH Measurement in Analytical Chemistry Labs
Learn about the significance of pH, the role of pH meters in measuring acidity/alkalinity, methods for determining pH levels in solutions, and the impact of pH on water quality and safety.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
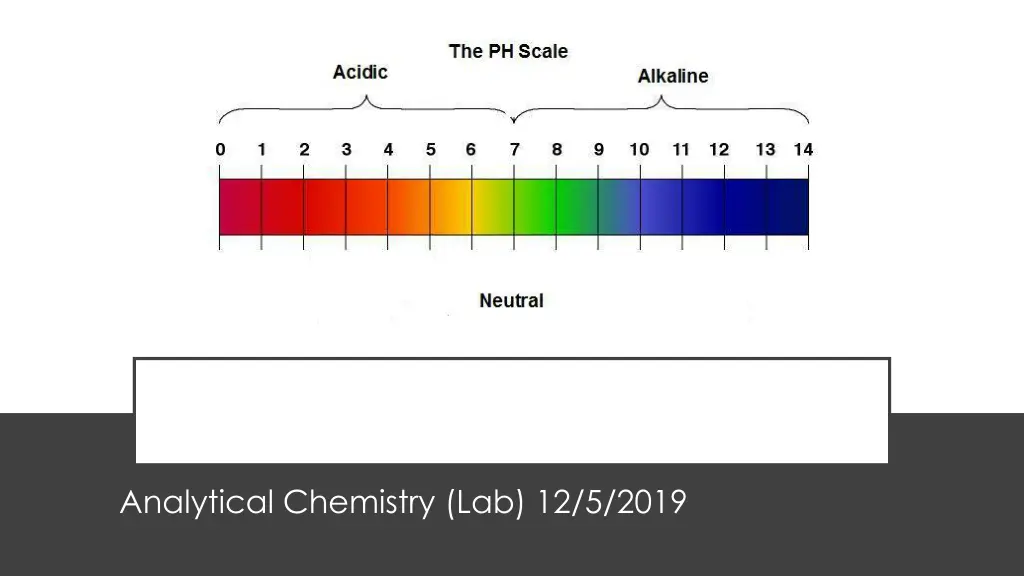
PH METER Analytical Chemistry (Lab) 12/5/2019
The pH of a solution is a measure of the molar concentration of hydrogen ions in the solution and as such is a measure of the acidity or basicity of the solution. The letters pH stand for "power of hydrogen" and the numerical value is defined as the negative base 10 logarithm of the molar concentration of hydrogen ions. pH= ???10[H+] PH
A pH meter is a device that measures the pH of a solution by measuring the voltage between two electrodes submerged in the solution. A pH meter is a scientific instrument that measures the hydrogen ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. PH METER DEFINITION The pH meter is used in many applications ranging from laboratory experimentation to quality control.
Although the pH of pure water is 7, drinking water and natural water exhibits a pH range because it contains dissolved minerals and gases. Surface waters typically range from pH 6.5 to 8.5 while groundwater ranges from pH 6 to 8.5. Water with a pH less than 6.5 is considered acidic. This water typically is corrosive and soft. It may contain metal ions, such as copper, iron, lead, manganese, and zinc. The metal ions may be toxic, may produce a metallic taste, and can stain fixtures and fabrics. The low pH can damage metal pipes and fixtures. Water with a pH higher than 8.5 is considered basic or alkaline. This water often is hard water, containing ions that can form scale deposits in pipes and contribute an alkali taste. PH OF DRINKING WATER
Methods for Finding PH of Solutions 1- pH indicators :Liquid acid-base indicators are weak organic acids or bases that present as different colors in their acid and base forms. An indicator has a specific pH range over which it changes from its acid form to its base form. pH test papers: is probably the most familiar pH paper. It is used to broadly test whether a solution is acidic or basic and comes in 3 types red, blue, and neutral. pH meters: is a device that measure a solution's pH by measuring the electrical potential difference between the pH electrode and a reference electrode.
An indicator has a specific pH range over which it changes from its acid form to its base form. An indicator is not useful outside its pH range because the indicator does not change color over these pH values. phenolphthalein (range pH 8.2 to 10.0; colorless to pink), bromthymol blue (range pH 6.0 to 7.6; yellow to blue), and litmus (range pH 4.5 to 8.3; red to blue). Universal indicators:are mixtures of several different pH indicators that extend the pH range over which they operate. Bogen universal indicator: PH INDICATORS
Liquid indicators are especially useful in acid-base titrations, where a noticeable pH change occurs near the equivalence point. Select a pH indicator whose pH range falls within the pH change of the reaction. pH indicators are also commonly used to perform quick checks on the pH of water samples (aquaria, pools, drinking water). This method of measuring pH is quick, inexpensive, and easy USES OF PH INDICATORS
Litmus paper: is probably the most familiar pH paper. Red litmus turns blue in basic solutions. Blue litmus turns red in acidic solution. Neutral litmus (usually purple) turns red in acidic solutions and blue in basic solutions. precise pH test papers or strips: There is a large selection of pH test papers and strips available, from wide pH range, low sensitivity to narrow pH range, high sensitivity. PH TEST PAPERS
There are many benefits to using pH strips and papers. Like indicator solutions, they are quick and easy to use Compared to pH meters, they are much less expensive. pH papers are portable, easy to store. well suited for field work. pH strips can be pasted into a lab notebook to retain experimental results pH strips can be pasted into a lab notebook to retain experimental results USES OF PH TEST PAPERS
PH meter, electric device used to measure hydrogen-ion activity (acidity or alkalinity) in solution. Fundamentally, a pH meter consists of a voltmeter attached to a pH-responsive electrode and a reference electrode. The pH-responsive electrode is usually glass, and the reference is usually a mercury mercurous chloride (calomel) electrode. When the two electrodes are immersed in a solution, they act as a battery.
Take the sample and use the pH meter to know the pH of the solution. Record all pH s and write the difference between them according to their pH either they are acidic or basic compound. PROCEDURE
Alkalosis: is a condition in which the body fluids have excess base (alkali). Respiratory alkalosis: is caused by a low carbon dioxide level in the blood. This can be due to: Fever- Being at a high altitude- Lack of oxygen-Liver disease-Lung disease, which causes you to breathe faster (hyperventilate) Metabolic alkalosis: is caused by too much bicarbonate in the blood. It can also occur due to certain kidney diseases. Hypochloremic alkalosis: is caused by an extreme lack or loss of chloride, such as from prolonged vomiting. Hypokalemic alkalosis: is caused by the kidneys' response to an extreme lack or loss of potassium. This can occur from taking certain water pills (diuretics). Compensated alkalosis: occurs when the body returns the acid-base balance to normal in cases of alkalosis, but bicarbonate and carbon dioxide levels remain abnormal. ALKALOSIS
Acidosis: is a condition in which there is too much acid in the body fluids. 1-Respiratory acidosis: develops when there is too much carbon dioxide (an acid) in the body. This type of acidosis is usually caused when the body is unable to remove enough carbon dioxide through breathing. Also Called hypercapnic acidosis and carbon dioxide acidosis. a)Chest deformities b)Chest injuries c)Chest muscle weakness d)Long-term (chronic) lung disease 2-Metabolic acidosis develops when too much acid is produced in the body. It can also occur when the kidneys cannot remove enough acid from the body. There are several types of metabolic acidosis: A-Diabetic acidosis (also called diabetic ketoacidosis and DKA) develops when substances called ketone bodies (which are acidic) build up during uncontrolled diabetes. B-Hyperchloremic acidosis is caused by the loss of too much sodium bicarbonate from the body, which can happen with severe diarrhea. 3-Lactic acidosis is a buildup of lactic acid. Lactic acid is mainly produced in muscle cells and red blood cells. It forms when the body breaks down carbohydrates to use for energy when oxygen levels are low. This can be caused by: a) Cancer b) Drinking too much alcohol c)Exercising vigorously for a very long time d)Liver failure e)Low blood sugar (hypoglycemia) ACIDOSIS